Chemistry:QTY Code
The QTY Code is a design method to transform membrane proteins that are intrinsically insoluble in water into variants with water solubility, while retaining their structure and function.
Similar structures of amino acids
The QTY Code is based on two key molecular structural facts: 1) all 20 natural amino acids are found in alpha-helices regardless of their chemical properties, although some amino acids have a higher propensity to form an alpha-helix; and, 2) several amino acids share striking structural similarities despite their very different chemical properties. These may be paired as: Glutamine (Q) vs Leucine (L); Threonine (T) vs Valine (V) and Isoleucine (I); and Tyrosine (Y) vs Phenylalanine (F).[1][2]
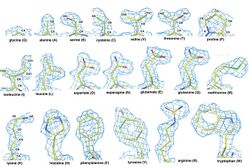
The QTY Code systematically replaces water-insoluble amino acids (L, V, I and F) with water-soluble amino acids (Q, T and Y) in transmembrane alpha-helices.[3] Thus, its application to membrane proteins changes the water-insoluble form of membrane proteins into water-soluble variants.[3][4] The QTY Code was specifically conceived to render G protein-coupled receptors (GPCRs) into a water-soluble form. Despite substantial transmembrane domain changes, the QTY variants of GPCRs maintain stable structure and ligand binding activities.[3][4][5][6][7]
File:H-bonds of N, Q, S, T, Y.tif
Hydrogen bond interactions between water and the amino acids
The side chain of glutamine (Q) can form 4 hydrogen bonds with 4 water molecules. There are 2 hydrogen donors from nitrogen and 2 hydrogen acceptors for oxygen. The –OH group of threonine (T) and tyrosine (Y) can form 3 hydrogen bonds with 3 water molecules (2 H-acceptors and 1 H-donor).[1] Color code: Green = carbon, red = oxygen, blue = nitrogen, gray = hydrogen, yellow disks = hydrogen bonds.

Three types of alpha-helices and with nearly identical molecular structure
There are 3 types of alpha-helices and with nearly identical molecular structure, namely: a) 1.5Å per amino acid rise, b) 100˚ per amino acid turn, c) 3.6 amino acids and 360˚ per helical turn, and d) 5.4Å per helical turn. The 3 types of alpha-helices are: 1) mostly hydrophobic amino acids including Leucine (L), Isoleucine (I), Valine (V), Phenylalanine (F), Methionine (M) and Alanine (A) that are commonly found as the helical transmembrane segments in membrane proteins; 2) mostly hydrophilic amino acids including Aspartic acid (D), Glutamic acid (E), Glutamine (Q), Lysine (K), Arginine (R), Serine (S), Threonine (T), Tyrosine (Y) that are commonly found on the out layer in water-soluble globular proteins; 3) mixed hydrophobic and hydrophilic amino acids that are partitioned in 2 faces: hydrophobic face and hydrophilic face, in an analogy, like our fingers with front and back. These alpha-helices sometimes attach to surface of membrane lipid bilayer, or partially buried to the hydrophobic core and partially close to the surface of water-soluble globular proteins.[2]
The QTY code
The QTY Code is likely universally applicable and also reversible, namely, Q changes to L, T changes to V and I, and Y changes to F. The QTY Code has been successful in designing many water-soluble variants of chemokine receptors and cytokine receptors. The QTY Code may likely be successfully applied to other water-insoluble aggregated proteins. The QTY Code is robust and straightforward: it is the simplest tool to carry out membrane protein design without sophisticated computer algorithms. Thus, it can be used broadly. The QTY Code has implications for designing additional GPCRs and other membrane proteins including cytokine receptors that are directly involved in cytokine storm syndrome.[3][4][5][6][7]
The QTY Code has also been applied to cytokine receptor water-soluble variants with the aim of combatting the cytokine storm syndrome (also called cytokine release syndrome) suffered by cancer patients receiving CAR-T therapy. This therapeutic application may be equally applicable to severely infected COVID-19 patients, for whom cytokine storms often lead to death.[7]
References
- ↑ 1.0 1.1 Stryer, Lubert (January 1, 1981). Biochemistry (2 ed.). W.H. Freeman and Company.
- ↑ 2.0 2.1 Branden, Carl Ivar; Tooze, John (January 1, 1999). Introduction to Protein Structure (2 ed.). Garland Science. ISBN 978-0-8153-2305-1.
- ↑ 3.0 3.1 3.2 3.3 Zhang, Shuguang; Tao, Fei; Qing, Rui; Tang, Hongzhi; Skuhersky, Michael; Corin, Karolina; Tegler, Lotta; Wassie, Asmamaw et al. (September 11, 2018). "QTY code enables design of detergent-free chemokine receptors that retain ligand-binding activities". PNAS 115 (37): E8652–E8659. doi:10.1073/pnas.1811031115. PMID 30154163. Bibcode: 2018PNAS..115E8652Z.
- ↑ 4.0 4.1 4.2 Qing, Rui; Skuhersky, Michael; Chung, Haeyoon; Badr, Myriam; Schubert, Thomas; Zhang, Shuguang (December 17, 2019). "QTY code designed thermostable and water-soluble chimeric chemokine receptors with tunable ligand affinity". PNAS 116 (51): 25668–25676. doi:10.1073/pnas.1909026116. PMID 31776256. Bibcode: 2019PNAS..11625668Q.
- ↑ 5.0 5.1 Hao, Shilei; Jin, David; Zhang, Shuguang; Qing, Rui (9 April 2020). "QTY Code-designed Water-soluble Fc-fusion Cytokine Receptors Bind to their Respective Ligands". QRB Discovery 1. doi:10.1017/qrd.2020.4. PMID 34192260.
- ↑ 6.0 6.1 Tegler, Lotta; Corin, Karolina; Pick, Horst; Brookes, Jennifer; Skuhersky, Michael; Vogel, Horst; Zhang, Shuguang (7 December 2020). "The G protein coupled receptor CXCR4 designed by the QTY code becomes more hydrophilic and retains cell signaling activity". Scientific Reports 10 (1): 21371. doi:10.1038/s41598-020-77659-x. PMID 33288780. Bibcode: 2020NatSR..1021371T.
- ↑ 7.0 7.1 7.2 Qing, Rui; Tao, Fei; Chatterjee, Pranam; Yang, Gaojie; Han, Qiuyi; Chung, Haeyoon; Ni, Jun; Suter, Bernhard et al. (18 December 2020). "Non-full-length Water-Soluble CXCR4QTY and CCR5QTY Chemokine Receptors: Implication for Overlooked Truncated but Functional Membrane Receptors". iScience 23 (12). doi:10.1016/j.isci.2020.101670. PMID 33376963. Bibcode: 2020iSci...23j1670Q.
Further reading
- Hung, Chien-Lun; Kuo, Yun-Hsuan; Lee, Su Wei; Chiang, Yun-Wei (2021). "Protein Stability Depends Critically on the Surface Hydrogen-Bonding Network: A Case Study of Bid Protein". The Journal of Physical Chemistry B 125 (30): 8373–8382. doi:10.1021/acs.jpcb.1c03245. PMID 34314184.
- Zayni, Sonja; Damiati, Samar; Moreno-Flores, Susana; Amman, Fabian; Hofacker, Ivo; Jin, David; Ehmoser, Eva-Kathrin (2021). "Enhancing the Cell-Free Expression of Native Membrane Proteins by in Silico Optimization of the Coding Sequence—An Experimental Study of the Human Voltage-Dependent Anion Channel". Membranes 11 (10): 741. doi:10.3390/membranes11100741. PMID 34677509.
- Root-Bernstein, Robert; Churchill, Beth (2021). "Co-Evolution of Opioid and Adrenergic Ligands and Receptors: Shared, Complementary Modules Explain Evolution of Functional Interactions and Suggest Novel Engineering Possibilities". Life 11 (11): 1217. doi:10.3390/life11111217. PMID 34833093. Bibcode: 2021Life...11.1217R.
- Vorobieva, Anastassia Andreevna (2021). "Principles and Methods in Computational Membrane Protein Design". Journal of Molecular Biology 433 (20). doi:10.1016/j.jmb.2021.167154. PMID 34271008.
- Martin, Joseph; Sawyer, Abigail (2019). "Elucidating the structure of membrane proteins". BioTechniques 66 (4): 167–170. doi:10.2144/btn-2019-0030. PMID 30987442.
- Tegler, Lotta; Corin, Karolina; Pick, Horst; Brookes, Jennifer; Skuhersky, Michael; Vogel, Horst; Zhang, Shuguang (2020). "The G protein coupled receptor CXCR4 designed by the QTY code becomes more hydrophilic and retains cell signaling activity". Scientific Reports 10 (1). doi:10.1038/s41598-020-77659-x. PMID 33288780. Bibcode: 2020NatSR..1021371T.
- Tao, Fei; Tang, Hongzhi; Zhang, Shuguang; Li, Mengke; Xu, Ping (2022). "Enabling QTY Server for Designing Water-Soluble α-Helical Transmembrane Proteins". mBio 13 (1): e0360421. doi:10.1128/mbio.03604-21. PMID 35038913.
- Smorodina, Eva; Tao, Fei; Qing, Rui; Jin, David; Yang, Steve; Zhang, Shuguang (2022). "Comparing 2 crystal structures and 12 AlphaFold2-predicted human membrane glucose transporters and their water-soluble QTY variants". QRB Discovery 3 (e5): e5. doi:10.1017/qrd.2022.6. PMID 37529287.
- Smorodina, Eva; Diankin, Igor; Tao, Fei; Qing, Rui; Yang, Steve; Zhang, Shuguang (2022). "Structural informatic study of determined and AlphaFold2 predicted molecular structures of 13 human solute carrier transporters and their water-soluble QTY variants". Scientific Reports 12 (1). doi:10.1038/s41598-022-23764-y. PMID 36418372. Bibcode: 2022NatSR..1220103S.
- Zhang, Shuguang; Egli, Martin (2022). "Hiding in plain sight: three chemically distinct α-helix types". Quarterly Reviews of Biophysics 55 (e7): e7. doi:10.1017/S0033583522000063. PMID 35722863.
- Qing, Rui; Hao, Shilei; Smorodina, Eva; Jin, David; Zalevsky, Arthur; Zhang, Shuguang (2022). "Protein Design: From the Aspect of Water Solubility and Stability". Chemical Reviews 122 (18): 14085–14179. doi:10.1021/acs.chemrev.1c00757. PMID 35921495.
- Meng, Run; Hao, Shilei; Sun, Changfa; Hou, Zongkun; Hou, Yao; Wang, Lili; Deng, Peiying; Deng, Jia et al. (2023). "Reverse-QTY code design of active human serum albumin self-assembled amphiphilic nanoparticles for effective anti-tumor drug doxorubicin release in mice". Proceedings of the National Academy of Sciences 120 (21). doi:10.1073/pnas.2220173120. PMID 37186820. Bibcode: 2023PNAS..12020173M.
- Qing, Rui; Xue, Mantian; Zhao, Jiayuan; Wu, Lidong; Breitwieser, Andreas; Smorodina, Eva; Schubert, Thomas; Azzellino, Giovanni et al. (2023). "Scalable biomimetic sensing system with membrane receptor dual-monolayer probe and graphene transistor arrays". Science Advances 9 (29). doi:10.1126/sciadv.adf1402. PMID 37478177. Bibcode: 2023SciA....9F1402Q.
- Sajeev-Sheeja, Akash; Smorodina, Eva; Zhang, Shuguang (2023). "Structural bioinformatics studies of bacterial outer membrane beta-barrel transporters and their AlphaFold2 predicted water-soluble QTY variants". PLOS ONE 18 (8). doi:10.1371/journal.pone.0290360. PMID 37607179. Bibcode: 2023PLoSO..1890360S.
- Li, Mengke; Wang, Yanze; Tao, Fei; Xu, Ping; Zhang, Shuguang (2023). "QTY code designed antibodies for aggregation prevention: A structural bioinformatic and computational study". Proteins: Structure, Function, and Bioinformatics 92 (2): 206–218. doi:10.1002/prot.26603. PMID 37795805.
- Li, Mengke; Qing, Rui; Tao, Fei; Xu, Ping; Zhang, Shuguang (2023). "Dynamic Dimerization of Chemokine Receptors and Potential Inhibitory Role of Their Truncated Isoforms Revealed through Combinatorial Prediction". International Journal of Molecular Sciences 24 (22). doi:10.3390/ijms242216266. PMID 38003455.
- Li, Mengke; Qing, Rui; Tao, Fei; Xu, Ping; Zhang, Shuguang (2024). "Inhibitory effect of truncated isoforms on GPCR dimerization predicted by combinatorial computational strategy". Computational and Structural Biotechnology Journal 23: 278–286. doi:10.1016/j.csbj.2023.12.008. PMID 38173876.
- Pan, Emily; Tao, Fei; Smorodina, Eva; Zhang, Shuguang (2024). "Structural bioinformatics studies of six human ABC transporters and their AlphaFold2-predicted water-soluble QTY variants". QRB Discovery 5. doi:10.1017/qrd.2024.2. PMID 38577032.
- Li, Mengke; Tang, Hongzhi; Qing, Rui; Wang, Yanze; Liu, Jiongqin; Wang, Rui; Lyu, Shan; Ma, Lina et al. (2024). "Design of a water-soluble transmembrane receptor kinase with intact molecular function by QTY code". Nature Communications 15 (1). doi:10.1038/s41467-024-48513-9. PMID 38858360. Bibcode: 2024NatCo..15.4293L.
- Karagöl, Taner; Karagöl, Alper; Zhang, Shuguang (2024). "Structural bioinformatics studies of serotonin, dopamine and norepinephrine transporters and their AlphaFold2 predicted water-soluble QTY variants and uncovering the natural mutations of L->Q, I->T, F->Y and Q->L, T->I and Y->F". PLOS ONE 19 (3). doi:10.1371/journal.pone.0300340. PMID 38517879. Bibcode: 2024PLoSO..1900340K.
- Karagöl, Alper; Karagöl, Taner; Smorodina, Eva; Zhang, Shuguang (2024). "Structural bioinformatics studies of glutamate transporters and their AlphaFold2 predicted water-soluble QTY variants and uncovering the natural mutations of L->Q, I->T, F->Y and Q->L, T->I, Y->F". PLOS ONE 19 (4). doi:10.1371/journal.pone.0289644. PMID 38598436. Bibcode: 2024PLoSO..1989644K.
- Karagöl, Alper; Karagöl, Taner; Zhang, Shuguang (2024). "Molecular Dynamic Simulations Reveal that Water-Soluble QTY-Variants of Glutamate Transporters EAA1, EAA2 and EAA3 Retain the Conformational Characteristics of Native Transporters". Pharmaceutical Research 41 (10): 1965–1977. doi:10.1007/s11095-024-03769-0. PMID 39322794.
- Smorodina, Eva; Tao, Fei; Qing, Rui; Yang, Steve; Zhang, Shuguang (2024). "Computational engineering of water-soluble human potassium ion channels through QTY transformation". Scientific Reports 14 (1): 28159. doi:10.1038/s41598-024-76603-7. PMID 39548172. Bibcode: 2024NatSR..1428159S.
- Sajeev‑Sheeja, Akash; Zhang, Shuguang (2024). "Structural molecular modeling of bacterial integral membrane protein enzymes and their AlphaFold2 predicted water‑soluble QTY variants". Journal of Proteins and Proteomics 15 (4): 635–645. doi:10.1007/s42485-024-00170-8.
- Johnsson, Finn; Karagöl, Taner; Karagöl, Alper; Zhang, Shuguang (2024). "Structural bioinformatic study of six human olfactory receptors and their AlphaFold3 predicted water‑soluble QTY variants and OR1A2 with an odorant octanoate and TAAR9 with spermidine". QRB Discovery 6. doi:10.1017/qrd.2024.18. PMID 39944883.
- Chen, Edward; Pan, Emily; Zhang, Shuguang (2025). "Structure Bioinformatics of Six Human Integral Transmembrane Enzymes and their AlphaFold3 Predicted Water‑Soluble QTY Analogs: Insights into FACE1 and STEA4 Binding Mechanisms". Pharmaceutical Research 42 (2): 291–305. doi:10.1007/s11095-025-03822-6. PMID 39966220.
- Chen, Edward; Zhang, Shuguang (2025). "Structural bioinformatic study of human mitochondrial respiratory integral membrane megacomplex and its AlphaFold3 predicted water‑soluble QTY megacomplex analog". QRB Discovery 6. doi:10.1017/qrd.2025.2. PMID 40160982.
- Wang, Jiayu; Pan, Emily; Zhang, Shuguang (2025). "Structural Bioinformatics Studies of Integral Transmembrane Enzymes pMMO Complex, C560, CYB, and DHSD and their AlphaFold3-Predicted Water-Soluble QTY Variants". BioCosmos: New Perspectives on the Origin and Evolution of Life 4 (1): 79–89. doi:10.2478/biocosmos-2024-0006. Bibcode: 2025BCos....4...79W.
 |
