Chemistry:Glutamine
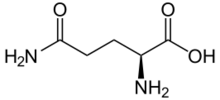 Skeletal formula of L-glutamine
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Glutamine
| |||
| Other names
L-Glutamine
(levo)glutamide 2,5-Diamino-5-oxopentanoic acid 2-Amino-4-carbamoylbutanoic acid Endari[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | Gln, Q | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties[2] | |||
| C5H10N2O3 | |||
| Molar mass | 146.146 g·mol−1 | ||
| Melting point | decomposes around 185°C | ||
| soluble | |||
| Acidity (pKa) | 2.2 (carboxyl), 9.1 (amino) | ||
Chiral rotation ([α]D)
|
+6.5º (H2O, c = 2) | ||
| Pharmacology | |||
| 1=ATC code }} | A16AA03 (WHO) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
| Clinical data | |
|---|---|
| Trade names | Endari, Nutrestore |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617035 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Gastrointestinal agent |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C5H10N2O3 |
| Molar mass | 146.146 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Glutamine (symbol Gln or Q)[4] is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases, and glutamine must be obtained from the diet.[5][6] It is encoded by the codons CAA and CAG.
In human blood, glutamine is the most abundant free amino acid.[7]
The dietary sources of glutamine include especially the protein-rich foods like beef, chicken, fish, dairy products, eggs, vegetables like beans, beets, cabbage, spinach, carrots, parsley, vegetable juices and also in wheat, papaya, Brussels sprouts, celery, kale and fermented foods like miso.
Functions
Glutamine plays a role in a variety of biochemical functions:
- Protein synthesis, as any other of the 20 proteinogenic amino acids
- Lipid synthesis, especially by cancer cells.[8]
- Regulation of acid-base balance in the kidney by producing ammonium[9]
- Cellular energy, as a source, next to glucose[10]
- Nitrogen donation for many anabolic processes, including the synthesis of purines[7]
- Carbon donation, as a source, refilling the citric acid cycle[11]
- Nontoxic transporter of ammonia in the blood circulation.[12]
- Integrity of healthy intestinal mucosa, though small randomized trials have shown no benefit in Crohn’s disease.[13]
Roles in metabolism
Glutamine maintains redox balance by participating in glutathione synthesis and contributing to anabolic processes such as lipid synthesis by reductive carboxylation.[14]
Glutamine provides a source of carbon and nitrogen for use in other metabolic processes. Glutamine is present in serum at higher concentrations than other amino acids[15] and is essential for many cellular functions. Examples include the synthesis of nucleotides and non-essential amino acids.[16] One of the most important functions of glutamine is its ability to be converted into α-KG, which helps to maintain the flow of the tricarboxylic acid cycle, generating ATP via the electron carriers NADH and FADH2.[17] The highest consumption of glutamine occurs in the cells of the intestines,[7] kidney cells (where it is used for acid-base balance), activated immune cells,[18] and many cancer cells.[8][11][19]
Production
Glutamine is produced industrially using mutants of Brevibacterium flavum, which gives ca. 40 g/L in 2 days using glucose as a carbon source.[20]
Biosynthesis
Glutamine synthesis from glutamate and ammonia is catalyzed by the enzyme glutamine synthetase. The majority of glutamine production occurs in muscle tissue, accounting for about 90% of all glutamine synthesized. Glutamine is also released, in small amounts, by the lungs and brain.[21] Although the liver is capable of glutamine synthesis, its role in glutamine metabolism is more regulatory than productive, as the liver takes up glutamine derived from the gut via the hepatic portal system.[7]
Uses
Nutrition
Glutamine is the most abundant naturally occurring, nonessential amino acid in the human body, and one of the few amino acids that can directly cross the blood–brain barrier.[7] Humans obtain glutamine through catabolism of proteins in foods they eat.[22] In states where tissue is being built or repaired, like growth of babies, or healing from wounds or severe illness, glutamine becomes conditionally essential.[22]
Sickle cell disease
In 2017, the U.S. Food and Drug Administration (FDA) approved L-glutamine oral powder, marketed as Endari, to reduce severe complications of sickle cell disease in people aged five years and older with the disorder.[1]
The safety and efficacy of L-glutamine oral powder were studied in a randomized trial of subjects ages five to 58 years old with sickle cell disease who had two or more painful crises within the 12 months prior to enrollment in the trial.[1] Subjects were assigned randomly to treatment with L-glutamine oral powder or placebo, and the effect of treatment was evaluated over 48 weeks.[1] Subjects who were treated with L-glutamine oral powder experienced fewer hospital visits for pain treated with a parenterally administered narcotic or ketorolac (sickle cell crises), on average, compared to subjects who received a placebo (median 3 vs. median 4), fewer hospitalizations for sickle cell pain (median 2 vs. median 3), and fewer days in the hospital (median 6.5 days vs. median 11 days).[1] Subjects who received L-glutamine oral powder also had fewer occurrences of acute chest syndrome (a life-threatening complication of sickle cell disease) compared with patients who received a placebo (8.6 percent vs. 23.1 percent).[1]
Common side effects of L-glutamine oral powder include constipation, nausea, headache, abdominal pain, cough, pain in the extremities, back pain and chest pain.[1]
L-glutamine oral powder received orphan drug designation.[1] The FDA granted the approval of Endari to Emmaus Medical Inc.[1]
Medical food
Glutamine is marketed as medical food and is prescribed when a medical professional believes a person in their care needs supplementary glutamine due to metabolic demands beyond what can be met by endogenous synthesis or diet.[23]
Safety
Glutamine is safe in adults and in preterm infants.[24] Although glutamine is metabolized to glutamate and ammonia, both of which have neurological effects, their concentrations are not increased much, and no adverse neurological effects were detected.[24] The observed safe level for supplemental L-glutamine in normal healthy adults is 14 g/day.[25]
Adverse effects of glutamine have been described for people receiving home parenteral nutrition and those with liver-function abnormalities.[26] Although glutamine has no effect on the proliferation of tumor cells, it is still possible that glutamine supplementation may be detrimental in some cancer types.[27]
Ceasing glutamine supplementation in people adapted to very high consumption may initiate a withdrawal effect, raising the risk of health problems such as infections or impaired integrity of the intestine.[27]
Structure
Glutamine can exist in either of two enantiomeric forms, L-glutamine and D-glutamine. The L-form is found in nature. Glutamine contains an α-amino group which is in the protonated −NH3+ form under biological conditions and a carboxylic acid group which is in the deprotonated −COO− form, known as carboxylate, under physiological conditions.
Research
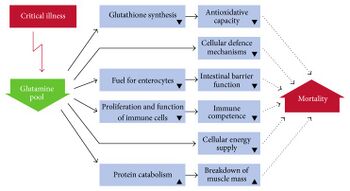
Glutamine mouthwash may be useful to prevent oral mucositis in people undergoing chemotherapy but intravenous glutamine does not appear useful to prevent mucositis in the GI tract.[29]
Glutamine supplementation was thought to have potential to reduce complications in people who are critically ill or who have had abdominal surgery but this was based on poor quality clinical trials.[30] Supplementation does not appear to be useful in adults or children with Crohn's disease or inflammatory bowel disease, but clinical studies as of 2016 were underpowered.[13] Supplementation does not appear to have an effect in infants with significant problems of the stomach or intestines.[31]
Some athletes use L-glutamine as supplement. Studies support the positive effects of the chronic oral administration of the supplement on the injury and inflammation induced by intense aerobic and exhaustive exercise, but the effects on muscle recovery from weight training are unclear.[32]
See also
- Isoglutamine
- Trinucleotide repeat disorder
- PolyQ tract
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "FDA approves new treatment for sickle cell disease". U.S. Food and Drug Administration (FDA) (Press release). 7 July 2017. Retrieved 10 July 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Weast, Robert C., ed (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-311. ISBN 0-8493-0462-8..
- ↑ "Glutamine Use During Pregnancy". 30 September 2019. https://www.drugs.com/pregnancy/glutamine.html.
- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html.
- ↑ Food and Nutrition Board of the Institute of Medicine (2006). "Protein and Amino Acids". Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, D.C.: National Academies Press. p. 147. ISBN 978-0-309-10091-5. http://www.nal.usda.gov/fnic/DRI/Essential_Guide/DRIEssentialGuideNutReq.pdf.
- ↑ "Is glutamine a conditionally essential amino acid?". Nutrition Reviews 48 (8): 297–309. August 1990. doi:10.1111/j.1753-4887.1990.tb02967.x. PMID 2080048.
- ↑ 7.0 7.1 7.2 7.3 7.4 "Interorgan amino acid transport and its regulation". The Journal of Nutrition 133 (6 Suppl 1): 2068S–2072S. June 2003. doi:10.1093/jn/133.6.2068S. PMID 12771367.

- ↑ 8.0 8.1 "Metabolic and mind shifts: from glucose to glutamine and acetate addictions in cancer". Current Opinion in Clinical Nutrition and Metabolic Care 18 (4): 346–353. July 2015. doi:10.1097/MCO.0000000000000178. PMID 26001655.
- ↑ Textbook of Medical Physiology (11th ed.). St. Louis, Mo: Elsevier Saunders. 2006. p. 393. ISBN 978-0-7216-0240-0.
- ↑ "Glutamine breakdown in rapidly dividing cells: waste or investment?". BioEssays 26 (7): 778–785. July 2004. doi:10.1002/bies.20063. PMID 15221859.
- ↑ 11.0 11.1 "Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells". The Journal of Cell Biology 178 (1): 93–105. July 2007. doi:10.1083/jcb.200703099. PMID 17606868.
- ↑ "Roles of Glutamate and Glutamine Transport in Ammonia Neurotoxicity: State of the Art and Question Marks". Endocrine, Metabolic & Immune Disorders Drug Targets 18 (4): 306–315. 2018. doi:10.2174/1871520618666171219124427. PMID 29256360.
- ↑ 13.0 13.1 "Dietary and enteral interventions for Crohn's disease". Current Opinion in Biotechnology 44: 69–73. April 2017. doi:10.1016/j.copbio.2016.11.011. PMID 27940405.
- ↑ "Reductive carboxylation supports redox homeostasis during anchorage-independent growth". Nature 532 (7598): 255–258. April 2016. doi:10.1038/nature17393. PMID 27049945. Bibcode: 2016Natur.532..255J.
- ↑ "Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney". Canadian Journal of Biochemistry 57 (3): 233–237. March 1979. doi:10.1139/o79-029. PMID 436006.
- ↑ "Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis". Proceedings of the National Academy of Sciences of the United States of America 104 (49): 19345–19350. December 2007. doi:10.1073/pnas.0709747104. PMID 18032601. Bibcode: 2007PNAS..10419345D.
- ↑ "The biology of cancer: metabolic reprogramming fuels cell growth and proliferation" (in English). Cell Metabolism 7 (1): 11–20. January 2008. doi:10.1016/j.cmet.2007.10.002. PMID 18177721.
- ↑ "Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?". The Journal of Nutrition 131 (9 Suppl): 2515S–2522S; discussion 2522S–4S. September 2001. doi:10.1093/jn/131.9.2515S. PMID 11533304.
- ↑ "Limits of aerobic metabolism in cancer cells" (in En). Scientific Reports 7 (1): 13488. October 2017. doi:10.1038/s41598-017-14071-y. PMID 29044214. Bibcode: 2017NatSR...713488F.
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2007. doi:10.1002/14356007.a02_057.pub2.
- ↑ "Glutamine and glutamate as vital metabolites". Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas 36 (2): 153–163. February 2003. doi:10.1590/S0100-879X2003000200002. PMID 12563517.
- ↑ 22.0 22.1 "Glutamine and glutamate: Nonessential or essential amino acids?". Animal Nutrition 1 (3): 119–122. September 2015. doi:10.1016/j.aninu.2015.08.008. PMID 29767158.
- ↑ "GlutaSolve, NutreStore, SYMPT-X G.I., SYMPT-X Glutamine (glutamine) Drug Side Effects, Interactions, and Medication Information on eMedicineHealth.". http://www.emedicinehealth.com/drug-glutamine/article_em.htm.
- ↑ 24.0 24.1 "Assessment of the safety of glutamine and other amino acids". The Journal of Nutrition 131 (9 Suppl): 2556S–2561S. September 2001. doi:10.1093/jn/131.9.2556S. PMID 11533313.
- ↑ "Risk assessment for the amino acids taurine, L-glutamine and L-arginine". Regulatory Toxicology and Pharmacology 50 (3): 376–399. April 2008. doi:10.1016/j.yrtph.2008.01.004. PMID 18325648.
- ↑ "Glutamine: commercially essential or conditionally essential? A critical appraisal of the human data". The American Journal of Clinical Nutrition 74 (1): 25–32. July 2001. doi:10.1093/ajcn/74.1.25. PMID 11451714.
- ↑ 27.0 27.1 "Side effects of long-term glutamine supplementation". Journal of Parenteral and Enteral Nutrition 37 (5): 607–616. September 2013. doi:10.1177/0148607112460682. PMID 22990615.
- ↑ "Glutamine: an obligatory parenteral nutrition substrate in critical care therapy". BioMed Research International 2015: 545467. 2015. doi:10.1155/2015/545467. PMID 26495301.
- ↑ "Nutrition in oncologic patients during antiblastic treatment". Frontiers in Bioscience 18 (1): 120–132. January 2013. doi:10.2741/4091. PMID 23276913.
- ↑ "Glutamine supplementation for critically ill adults". The Cochrane Database of Systematic Reviews 2018 (9): CD010050. September 2014. doi:10.1002/14651858.CD010050.pub2. PMID 25199493.
- ↑ "Glutamine supplementation to prevent morbidity and mortality in preterm infants". The Cochrane Database of Systematic Reviews 4 (4): CD001457. April 2016. doi:10.1002/14651858.CD001457.pub6. PMID 27089158.
- ↑ "Role of glutamine, as free or dipeptide form, on muscle recovery from resistance training: a review study". Nutrire 43 (1): 28. 5 December 2018. doi:10.1186/s41110-018-0087-9. ISSN 2316-7874.
External links
- Glutamine spectra acquired through mass spectroscopy
- "Glutamine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/56-85-9.
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |