Chemistry:Rubidium hydrogen sulfate
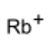 rubidium ion
| |
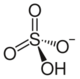 hydrogen sulfate ion
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| RbHSO4 | |
| Molar mass | 182,54 g/mol−1 |
| Appearance | Crystals with no colour[1] |
| Density | 2.89 g·cm−3 |
| Melting point | 214 °C (417 °F; 487 K)[2] |
| Related compounds | |
Other cations
|
rubidium oxide rubidium hydroxide |
Related compounds
|
rubidium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rubidium hydrogen sulfate, sometimes referred to as rubidium bisulfate, is the half neutralized rubidium salt of sulfuric acid. It has the formula RbHSO4.
Synthesis
It may be synthesised with water and a stoichiometric amount of rubidium disulfate. Reaction takes place where there is no humidity:[3]
- [math]\ce{ {Rb2S2O7} + H2O -> 2RbHSO_4 }[/math]
There is another method of creation. It is similar to the synthesis of sodium sulfate and potassium sulfate. This reaction requires rubidium chloride and a little bit of warm sulfuric acid. Some hydrogen chloride is also produced during the reaction.
- [math]\ce{ {H2SO4} + RbCl -> {RbHSO4} + HCl }[/math]
Properties
It is a hygroscopic compound. It has a monoclinic crystal structure, its structure is P21/n. Dimensions of the unit cell are: a = 1440 pm, b = 462.2 pm, c = 1436 pm and β = 118.0°. Its crystals are isomorphs with ammonium hydrogen sulfate crystals.[4]
Its standard enthalpy is −1166 kJ/mol.[5] During its dissolution in water, there is 15.62 kJ/mol energy produced.[6]
After warming up it decomposes to rubidium disulfate and water:[7]
- [math]\ce{ 2RbHSO4 -> Rb2S2O7 + H2O }[/math]
Like potassium and caesium, rubidium has another hydrogen sulfate compound as well: Rb3H(SO4)2.
References
- ↑ Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN:978-3-5406-0035-0, S. 692 (Taschenbuch für Chemiker und Physiker, p. 692, at Google Books).
- ↑ R. Fehrmann, S. Boghosian, H. Hamma-Cugny, J. Rogez: "Phase diagrams, structural and thermodynamic properties of molten salt solvents for the industrial SO2-oxidation catalyst" Abstract
- ↑ S. B. Rasmussen, H. Hamma, K. M. Eriksen, G. Hatem, M. Gaune-Escard, R. Fehrmann: "Physico-chemical properties and transition metal complex formation in alkali pyrosulfate and hydrogen sulfate melts". VII International Conference on Molten Slags Fluxes and Salts, The South African Institute of Mining and Metallurgy, 2004. Volltext (PDF; 661 kB)
- ↑ J. P. Ashmore, H. E. Petch: "The Structure of RbHSO4 in its Paraelectric Phase" in Can. J. Phys 1975, 53(24), S. 2694-2702. doi:10.1139/p75-328
- ↑ L. A. Cowan, R. M. Morcos, N. Hatada, A. Navrotsky, S. M. Haile: "High temperature properties of Rb3H(SO4)2 at ambient pressure: Absence of a polymorphic, superprotonic transition" in Solid State Ionics 2008, 179, S. 305-313. Volltext (PDF; 837 kB)
- ↑ M. de Forcrand: "Sur les chlorures et sulfates de rubidium et de caesium" in Compt. Rend. Hebd. 1906, 143, S. 98. Volltext
- ↑ R. Abegg, F. Auerbach: "Handbuch der anorganischen Chemie". Verlag S. Hirzel, Bd. 2, 1908. S. 432.Volltext
 |

