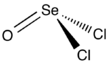
Chemistry:Selenium oxydichloride

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Selenium oxychloride
| |||
| Other names
Seleninyl chloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| SeOCl2 | |||
| Molar mass | 165.87 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 2.43 g/cm3, liquid | ||
| Melting point | 10.9 °C (51.6 °F; 284.0 K) | ||
| Boiling point | 177.2 °C (351.0 °F; 450.3 K) | ||
Refractive index (nD)
|
1.651 (20 °C) | ||
| Structure | |||
| trigonal pyramidal | |||
| Hazards | |||
| GHS pictograms |    
| ||
| GHS Signal word | Warning | ||
| H301, H314, H331, H373, H410 | |||
| P260, P261, P264, P270, P271, P273, P280, P301+310, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P311, P314, P321, P330, P363, P391, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published)
|
2 mg/kg (rabbit, dermal)[1] | ||
| Related compounds | |||
Related compounds
|
SOCl2, POCl3 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Selenium oxydichloride is the inorganic compound with the formula SeOCl2. It is a colorless liquid. With a high dielectric constant (55) and high specific conductance, it is an attractive solvent. Structurally, it is a close chemical relative of thionyl chloride SOCl2, being a pyramidal molecule.
Preparation and reactions
Selenium oxydichloride can be prepared by several methods, and a common one involves the conversion of selenium dioxide to dichloroselenious acid followed by dehydration:[3]
- SeO2 + 2 HCl → Se(OH)2Cl2
- Se(OH)2Cl2 → SeOCl2 + H2O
The original synthesis involved the redistribution reaction of selenium dioxide and selenium tetrachloride.
Pure selenium oxydichloride autoionizes to a dimer:[4]
- SeOCl2 ↔ (SeO)2Cl+3 + Cl−
The SeOCl2 is generally a labile Lewis acid and solutions of sulfur trioxide in SeOCl2 likely form [SeOCl]+[SO3Cl]− the same way.[5]
The compound hydrolyzes readily to form hydrogen chloride and selenium dioxide,[citation needed] and very few organic compounds dissolve in it without reaction. At elevated temperatures, it is a strong oxidizer, yielding a chloride, selenium dioxide, and diselenium dichloride.[6]
See also
- Selenium oxybromide (SeOBr2)
- Selenous acid (H2SeO3)
References
- ↑ "Selenium compounds (as Se)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/7782492.html.
- ↑ "Selenium oxychloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/24647#section=Safety-and-Hazards.
- ↑ Smith, G. B. L.; Jackson, Julius (1950). "Selenium(IV) Oxychloride". Inorganic Syntheses. 3. pp. 130–137. doi:10.1002/9780470132340.ch34. ISBN 9780470132340.
- ↑ Audrieth & Kleinberg 1953, p. 237.
- ↑ Audrieth & Kleinberg 1953, pp. 239–242.
- ↑ Audrieth, Ludwig F.; Kleinberg, Jacob (1953). Non-aqueous solvents. New York: John Wiley & Sons. pp. 235–6. https://archive.org/details/cftri.2662nonaqueoussolven0000ludw/page/.
 |