Chemistry:Selenocysteine
| |
| |
| Names | |
|---|---|
| IUPAC name
Selenocysteine
| |
| Systematic IUPAC name
3-Selanyl-L-alanine (semisystematic name)
2-Amino-3-selanylpropanoic acid (fully systematic name) | |
| Other names
L-Selenocysteine; Selenium-cysteine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H7NO2Se | |
| Molar mass | 168.065 g·mol−1 |
| Properties | |
| Acidity (pKa) | 5.43[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Selenocysteine (symbol Sec or U,[3] in older publications also as Se-Cys)[4] is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the sulfur.
Selenocysteine is present in several enzymes (for example glutathione peroxidases, tetraiodothyronine 5′ deiodinases, thioredoxin reductases, formate dehydrogenases, glycine reductases, selenophosphate synthetase 2, methionine-R-sulfoxide reductase B1 (SEPX1), and some hydrogenases). It occurs in all three domains of life, including important enzymes (listed above) present in humans.[5]
Selenocysteine was discovered in 1974[6] by biochemist Thressa Stadtman at the National Institutes of Health.[7]
Chemistry
Selenocysteine is the Se-analogue of cysteine. It is rarely encountered outside of living tissue (and is not available commercially) because it is very susceptible to air-oxidation. More common is the oxidized derivative selenocystine, which has an Se-Se bond.[8] Both selenocysteine and selenocystine are white solids. The Se-H group is more acidic (pKa = 5.43[2]) than the thiol group; thus, it is deprotonated at physiological pH.[9]
Structure
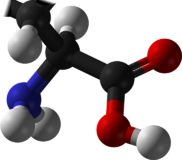
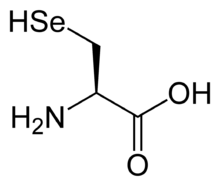
Selenocysteine has the same structure as cysteine, but with an atom of selenium taking the place of the usual sulfur. It has a selenol group. Like other natural proteinogenic amino acids, cysteine and selenocysteine have L chirality in the older D/L notation based on homology to D- and L-glyceraldehyde. In the newer R/S system of designating chirality, based on the atomic numbers of atoms near the asymmetric carbon, they have R chirality, because of the presence of sulfur or selenium as a second neighbor to the asymmetric carbon. The remaining chiral amino acids, having only lighter atoms in that position, have S chirality.)
Proteins which contain a selenocysteine residue are called selenoproteins. Most selenoproteins contain a single selenocysteine residue. Selenoproteins that exhibit catalytic activity are called selenoenzymes.[10]
Biology
Selenocysteine has a lower reduction potential than cysteine. These properties make it very suitable in proteins that are involved in antioxidant activity.[11]
Although it is found in the three domains of life, it is not universal in all organisms.[12] Unlike other amino acids present in biological proteins, selenocysteine is not coded for directly in the genetic code.[13] Instead, it is encoded in a special way by a UGA codon, which is normally the "opal" stop codon. Such a mechanism is called translational recoding[14] and its efficiency depends on the selenoprotein being synthesized and on translation initiation factors.[15] When cells are grown in the absence of selenium, translation of selenoproteins terminates at the UGA codon, resulting in a truncated, nonfunctional enzyme. The UGA codon is made to encode selenocysteine by the presence of a selenocysteine insertion sequence (SECIS) in the mRNA. The SECIS element is defined by characteristic nucleotide sequences and secondary structure base-pairing patterns. In bacteria, the SECIS element is typically located immediately following the UGA codon within the reading frame for the selenoprotein.[16] In Archaea and in eukaryotes, the SECIS element is in the 3′ untranslated region (3′ UTR) of the mRNA and can direct multiple UGA codons to encode selenocysteine residues.[17]
Unlike the other amino acids, no free pool of selenocysteine exists in the cell. Its high reactivity would cause damage to cells.[18] Instead, cells store selenium in the less reactive oxidized form, selenocystine, or in methylated form, selenomethionine. Selenocysteine synthesis occurs on a specialized tRNA, which also functions to incorporate it into nascent polypeptides.
The primary and secondary structure of selenocysteine-specific tRNA, tRNASec, differ from those of standard tRNAs in several respects, most notably in having an 8-base-pair (bacteria) or 10-base-pair (eukaryotes)[Archaea?] acceptor stem, a long variable region arm, and substitutions at several well-conserved base positions. The selenocysteine tRNAs are initially charged with serine by seryl-tRNA ligase, but the resulting Ser-tRNASec is not used for translation because it is not recognised by the normal translation elongation factor (EF-Tu in bacteria, eEF1A in eukaryotes).[Archaea?]
Rather, the tRNA-bound seryl residue is converted to a selenocysteine residue by the pyridoxal phosphate-containing enzyme selenocysteine synthase. In eukaryotes and archaea, two enzymes are required to convert tRNA-bound seryl residue into tRNA selenocysteinyl residue: PSTK (O-phosphoseryl-tRNA[Ser]Sec kinase) and selenocysteine synthase.[19][20] Finally, the resulting Sec-tRNASec is specifically bound to an alternative translational elongation factor (SelB or mSelB (or eEFSec)), which delivers it in a targeted manner to the ribosomes translating mRNAs for selenoproteins. The specificity of this delivery mechanism is brought about by the presence of an extra protein domain (in bacteria, SelB) or an extra subunit (SBP2 for eukaryotic mSelB/eEFSec)[Archaea?] which bind to the corresponding RNA secondary structures formed by the SECIS elements in selenoprotein mRNAs.
Selenocysteine is decomposed by the enzyme selenocysteine lyase into L-alanine and selenide.[21]
(As of 2021), 136 human proteins (in 37 families) are known to contain selenocysteine (selenoproteins).[22]
Selenocysteine derivatives γ-glutamyl-Se-methylselenocysteine and Se-methylselenocysteine occur naturally in plants of the genera Allium and Brassica.[23]
Applications
Biotechnological applications of selenocysteine include use of 73Se-labeled Sec (half-life of 73Se = 7.2 hours) in positron emission tomography (PET) studies and 75Se-labeled Sec (half-life of 75Se = 118.5 days) in specific radiolabeling, facilitation of phase determination by multiwavelength anomalous diffraction in X-ray crystallography of proteins by introducing Sec alone, or Sec together with selenomethionine (SeMet), and incorporation of the stable 77Se isotope, which has a nuclear spin of 1/2 and can be used for high-resolution NMR, among others.[5]
See also
- Pyrrolysine, another amino acid not in the basic set of 20.
- Selenomethionine, another selenium-containing amino acid, which is randomly substituted for methionine.
References
- ↑ Merck Index, 12th Edition, 8584
- ↑ 2.0 2.1 Thapa, Bishnu; Schlegel, H. Bernhard (2016-11-10). "Theoretical Calculation of p K a 's of Selenols in Aqueous Solution Using an Implicit Solvation Model and Explicit Water Molecules" (in en). The Journal of Physical Chemistry A 120 (44): 8916–8922. doi:10.1021/acs.jpca.6b09520. ISSN 1089-5639. PMID 27748600. https://pubs.acs.org/doi/10.1021/acs.jpca.6b09520.
- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html.
- ↑ "IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN) and Nomenclature Committee of IUBMB (NC-IUBMB)". European Journal of Biochemistry 264 (2): 607–609. 17 August 1999. doi:10.1046/j.1432-1327.1999.news99.x.
- ↑ 5.0 5.1 "Selenocysteine in proteins—properties and biotechnological use". Biochimica et Biophysica Acta (BBA) - General Subjects 1726 (1): 1–13. October 2005. doi:10.1016/j.bbagen.2005.05.010. PMID 15967579.
- ↑ "Stadtman Pioneer of Selenium Biochemistry - Office of NIH History and Stetten Museum". https://history.nih.gov/display/history/Stadtman+Pioneer+of+Selenium+Biochemistry.
- ↑ "Selenium biochemistry". Science 183 (4128): 915–22. March 1974. doi:10.1126/science.183.4128.915. PMID 4605100. Bibcode: 1974Sci...183..915S.
- ↑ Görbitz, C. H.; Levchenko, V.; Semjonovs, J.; Sharif, M. Y. (2015). "Crystal structure of seleno-L-cystine dihydrochloride". Acta Crystallogr. E 71 (6): 726–729. doi:10.1107/S205698901501021X. PMID 26090162.
- ↑ "Why nature chose selenium". ACS Chemical Biology 11 (4): 821–841. April 2016. doi:10.1021/acschembio.6b00031. PMID 26949981.
- ↑ "Selenium-containing enzymes in mammals: chemical perspectives". Journal of Chemical Sciences 117 (4): 287–303. 2005. doi:10.1007/BF02708441. http://repository.ias.ac.in/79332/1/53-PUB.pdf.
- ↑ "Conformational preferences and pK(a) value of selenocysteine residue". Biopolymers 95 (5): 345–53. May 2011. doi:10.1002/bip.21581. PMID 21213257.
- ↑ "A forgotten debate: is selenocysteine the 21st amino acid?". Journal of the National Cancer Institute 96 (7): 504–5. April 2004. doi:10.1093/jnci/96.7.504. PMID 15069108.
- ↑ "Selenoprotein synthesis: an expansion of the genetic code". Trends in Biochemical Sciences 16 (12): 463–7. December 1991. doi:10.1016/0968-0004(91)90180-4. PMID 1838215.
- ↑ "Recoding: translational bifurcations in gene expression". Gene 286 (2): 187–201. March 2002. doi:10.1016/S0378-1119(02)00423-7. PMID 11943474.
- ↑ "The efficiency of selenocysteine incorporation is regulated by translation initiation factors". Journal of Molecular Biology 400 (4): 659–64. July 2010. doi:10.1016/j.jmb.2010.05.026. PMID 20488192.
- ↑ Atkins, J. F. (2009). Recoding: Expansion of Decoding Rules Enriches Gene Expression. Springer. p. 31. ISBN 978-0-387-89381-5. https://books.google.com/books?id=8cSZpPWXoqIC&pg=PA31.
- ↑ "Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons". The EMBO Journal 12 (8): 3315–22. August 1993. doi:10.1002/j.1460-2075.1993.tb06001.x. PMID 8344267.
- ↑ Spallholz, Julian E. (July 1994). "On the nature of selenium toxicity and carcinostatic activity" (in en). Free Radical Biology and Medicine 17 (1): 45–64. doi:10.1016/0891-5849(94)90007-8. PMID 7959166. https://linkinghub.elsevier.com/retrieve/pii/0891584994900078.
- ↑ "Biosynthesis of selenocysteine on its tRNA in eukaryotes". PLOS Biology 5 (1): e4. January 2007. doi:10.1371/journal.pbio.0050004. PMID 17194211.
- ↑ "RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea". Proceedings of the National Academy of Sciences of the United States of America 103 (50): 18923–7. December 2006. doi:10.1073/pnas.0609703104. PMID 17142313. Bibcode: 2006PNAS..10318923Y.
- ↑ "Selenoproteins: molecular pathways and physiological roles". Physiological Reviews 94 (3): 739–77. July 2014. doi:10.1152/physrev.00039.2013. PMID 24987004.
- ↑ "SelenoDB 2.0: annotation of selenoprotein genes in animals and their genetic diversity in humans". Nucleic Acids Research 42 (Database issue): D437-43. January 2014. doi:10.1093/nar/gkt1045. PMID 24194593. [1]
- ↑ Block, E. (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 978-0-85404-190-9. https://books.google.com/books?id=6AB89RHV9ucC.
Further reading
- "Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America 83 (13): 4650–4. July 1986. doi:10.1073/pnas.83.13.4650. PMID 2941757. Bibcode: 1986PNAS...83.4650Z.
- "Cotranslational insertion of selenocysteine into formate dehydrogenase from Escherichia coli directed by a UGA codon". Proceedings of the National Academy of Sciences of the United States of America 84 (10): 3156–60. May 1987. doi:10.1073/pnas.84.10.3156. PMID 3033637. Bibcode: 1987PNAS...84.3156Z.
- "Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety". Proceedings of the National Academy of Sciences of the United States of America 73 (8): 2659–63. August 1976. doi:10.1073/pnas.73.8.2659. PMID 1066676. Bibcode: 1976PNAS...73.2659C.
- "Selenocysteine: Wherefore Art Thou?". Journal of Proteome Research 15 (2): 677–8. February 2016. doi:10.1021/acs.jproteome.5b01028. PMID 26680273.
External links
 |