Chemistry:Seyferth–Gilbert homologation
| Seyferth–Gilbert homologation | |
|---|---|
| Named after | Dietmar Seyferth John C. Gilbert |
| Reaction type | Homologation reaction |
| Identifiers | |
| Organic Chemistry Portal | seyferth-gilbert-homologation |
| RSC ontology ID | RXNO:0000387 |
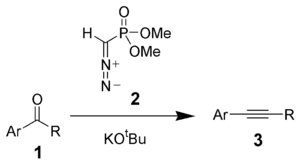
The Seyferth–Gilbert homologation is a chemical reaction of an aryl ketone 1 (or aldehyde) with dimethyl (diazomethyl)phosphonate 2 and potassium tert-butoxide to give substituted alkynes 3.[1][2] Dimethyl (diazomethyl)phosphonate 2 is often called the Seyferth–Gilbert reagent.[3]
This reaction is called a homologation because the product has exactly one additional carbon more than the starting material.
Reaction mechanism
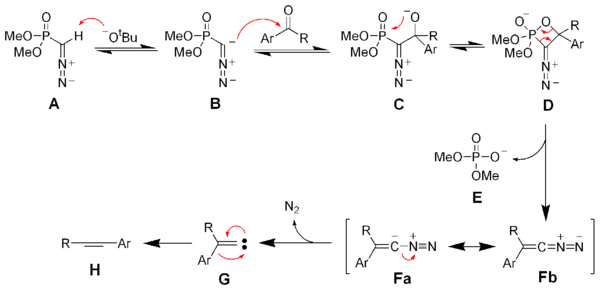
Deprotonation of the Seyferth–Gilbert reagent A gives an anion B, which reacts with the ketone to form the oxaphosphetane D. Elimination of dimethylphosphate E gives the vinyl diazo-intermediate Fa and Fb. The generation of nitrogen gas gives a vinyl carbene G, which via a 1,2-migration forms the desired alkyne H.
Bestmann modification
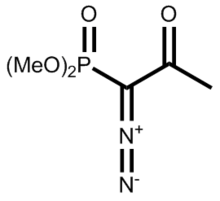
| |
| Names | |
|---|---|
| IUPAC name
dimethyl (1-diazo-2-oxopropyl)phosphonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H9N2O4P | |
| Molar mass | 192.11 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
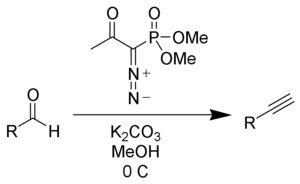
The dimethyl (diazomethyl)phosphonate carbanion can be generated in situ from dimethyl-1-diazo-2-oxopropylphosphonate (also called the Ohira-Bestmann reagent) by reaction with methanol and potassium carbonate as the base by cleavage of the acetyl group as methyl acetate. Reaction of Bestmann's reagent with aldehydes gives terminal alkynes often in very high yield and fewer steps than the Corey–Fuchs reaction.[4][5]
The use of the milder potassium carbonate makes this procedure much more compatible with a wide variety of functional groups.
Improved in situ generation of the Ohira-Bestmann reagent
Recently a safer and more scalable approach has been developed for the synthesis of alkynes from aldehydes. This protocol takes advantage of a stable sulfonyl azide, rather than tosyl azide, for the in situ generation of the Ohira−Bestmann reagent.[6]
Other modifications
Another modification for less reactive aldehydes is made by replacement of potassium carbonate with caesium carbonate in MeOH and results in a drastic[quantify] yield increase.[7]
See also
References
- ↑ D. Seyferth; R. S. Marmor; P. Hilbert (1971). "Reactions of dimethylphosphono-substituted diazoalkanes. (MeO)2P(O)CR transfer to olefins and 1,3-dipolar additions of (MeO)2P(O)C(N2)R". J. Org. Chem. 36 (10): 1379–1386. doi:10.1021/jo00809a014.
- ↑ J. C. Gilbert; U. Weerasooriya (1982). "Diazoethenes: their attempted synthesis from aldehydes and aromatic ketones by way of the Horner-Emmons modification of the Wittig reaction. A facile synthesis of alkynes". J. Org. Chem. 47 (10): 1837–1845. doi:10.1021/jo00349a007.
- ↑ D. G. Brown; E. J. Velthuisen; J. R. Commerford; R. G. Brisbois; T. H. Hoye (1996). "A Convenient Synthesis of Dimethyl (Diazomethyl)phosphonate (Seyferth/Gilbert Reagent)". J. Org. Chem. 61 (7): 2540–2541. doi:10.1021/jo951944n.
- ↑ S. Müller; B. Liepold; G. Roth; H. J. Bestmann (1996). "An Improved One-pot Procedure for the Synthesis of Alkynes from Aldehydes". Synlett 1996 (6): 521–522. doi:10.1055/s-1996-5474.
- ↑ G. Roth; B. Liepold; S. Müller; H. J. Bestmann (2004). "Further Improvements of the Synthesis of Alkynes from Aldehydes". Synthesis 2004 (1): 59–62. doi:10.1055/s-2003-44346.
- ↑ Jepsen, T.H, Kristensen, J.L. J. Org. Chem. 2014, "In Situ Generation of the Ohira–Bestmann Reagent from Stable Sulfonyl Azide: Scalable Synthesis of Alkynes from Aldehydes". http://pubs.acs.org/doi/abs/10.1021/jo501803f
- ↑ Lidija Bondarenko; Ina Dix; Heino Hinrichs; Henning Hopf (2004). "Cyclophanes. Part LII:1 Ethynyl[2.2]paracyclophanes – New Building Blocks for Molecular Scaffolding". Synthesis 2004 (16): 2751–2759. doi:10.1055/s-2004-834872.
 |