Chemistry:Sodium glycerophosphate
 | |
| Clinical data | |
|---|---|
| Trade names | Glycophos |
| AHFS/Drugs.com | Professional Drug Facts |
| Pregnancy category |
|
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C3H7Na2O6P |
| Molar mass | 216.036 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 98 to 100 °C (208 to 212 °F) |
| Solubility in water | very soluble |
| |
| |
Sodium glycerophosphate, sold under the brand name Glycophos, is a medication used to supplement phosphate.[1][2] It is administered via intravenous infusion.[1][2]
Sodium glycerophosphate is an organic phosphate salt.[1][2]
It was approved for medical use in Australia in November 2019.[3][1][4]
It is an unapproved medication in the United States that was used as a substitute for inorganic phosphate during a drug shortage.[2]
Chemistry
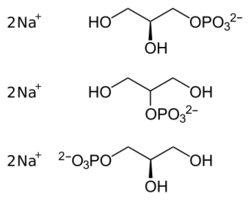
The substance is a mixture of disodium glycerol 1- and 2-phosphates, which have different amounts of water of crystallization; the total amount is 5 1⁄2 H2O per glycerol phosphate molecule. It is a white to off-white powder which may or may not be crystalline, has no discernible odor and tastes salty. It melts at 98 to 100 °C (208 to 212 °F) and decomposes at 130 °C (266 °F). Aqueous solutions have a pH of about 9.5.[5]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 "ARTG Entry:312021 Glycophos Product Information" (PDF). http://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2019-PI-02257-1.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 "Glycophos- sodium glycolate injection, solution". 16 January 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dcd92dc7-c6f9-4866-9edd-c95d5097c4e5.
- ↑ "Glycophos Australian prescription medicine decision summary". https://www.tga.gov.au/apm-summary/glycophos.
- ↑ "Summary for ARTG Entry:312021 Glycophos sodium glycerophosphate (as hydrate) 4.32 g/20 mL concentrated solution for injection ampoule". https://tga-search.clients.funnelback.com/s/search.html?collection=tga-artg&profile=record&meta_i=312021.
- ↑ (in de) Hagers Handbuch der pharmazeutischen Praxis. VI.A (4th ed.). Springer. 15 August 1977. pp. 91–92. ISBN 3-540-05123-6.
Further reading
- "Australian Public Assessment Report for Sodium glycerophosphate (as hydrate)". https://www.tga.gov.au/sites/default/files/auspar-sodium-glycerophosphate-200213.pdf.
External links
- "Sodium glycerophosphate anhydrous". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/1334-74-3.
 |

