Chemistry:Stichodactyla toxin
| ShK domain-like | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | ShK | ||||||||
| Pfam | PF01549 | ||||||||
| InterPro | IPR003582 | ||||||||
| SMART | SM00254 | ||||||||
| SCOP2 | 1roo / SCOPe / SUPFAM | ||||||||
| TCDB | 8.B.14 | ||||||||
| OPM superfamily | 296 | ||||||||
| OPM protein | 2lg4 | ||||||||
| |||||||||
| Kappa-stichotoxin-She3a | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | ? | ||||||
| UniProt | P29187 | ||||||
| |||||||
Stichodactyla toxin (ShK, ShkT) is a 35-residue basic peptide from the sea anemone Stichodactyla helianthus that blocks a number of potassium channels. Related peptides form a conserved family of protein domains known as the ShkT domain. Another well-studied toxin of the family is BgK from Bunodosoma granulifera.
An analogue of ShK called ShK-186 or Dalazatide is in human trials as a therapeutic for autoimmune diseases.
History
Stichodactyla helianthus is a species of sea anemone (Phylum: Cnidaria) belonging to the family Stichodactylidae. Helianthus comes from the Greek words helios meaning sun, and anthos meaning flower, which corresponds to the species' common name "sun anemone". It is sessile and uses potent neurotoxins for defense against its primary predator, the spiny lobster.[1] The venom contains, among other components, numerous ion channel-blocking peptides. In 1995, a group led by Olga Castaneda and Evert Karlsson isolated ShK, a potassium channel-blocking 35-residue peptide from S. helianthus.[2] The same year, William Kem and his collaborator Michael Pennington synthesized and folded ShK, and showed it blocked neuronal and lymphocyte voltage-dependent potassium channels.[3] In 1996, Ray Norton determined the three-dimensional structure of ShK.[4] In 2005–2006, George Chandy, Christine Beeton and Michael Pennington developed ShK-170 and ShK-186 (ShK-L5), selective blockers of Kv1.3.[5][6] ShK-186, now called Dalazatide, was advanced to human trials in 2015-2017 by Shawn Iadonato and Eric Tarcha, as the first-in-man Kv1.3 blocker for autoimmune disease.[7]
Structure
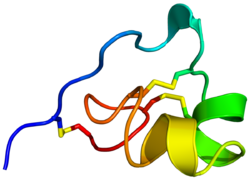
File:ShK toxin Fig 3.tiff ShK is cross-linked by three disulfide bridges: Cys3-Cys35, Cys12-Cys28, and Cys17-Cys32. The solution structure of ShK reveals two short α-helices comprising residues 14-19 and 21–24; the N-terminal eight residues adopt an extended conformation, followed by a pair of interlocking turns that resemble a 310 helix; the C-terminal Cys35 residue forms a nearly head-to-tail cyclic structure through a disulfide bond with Cys3.[4][8][9][10][11][12]
Phylogenetic relationships of ShK and ShK domains
File:ShK toxin Fig 4.tiff The SMART database at the EMBL, as of May 2018,[13] lists 3345 protein domains with structural resemblance to ShK in 1797 proteins (1 to 8 domains/protein), many in the worm Caenorhabditis elegans and venomous snakes.[14][15][16][17][18] The majority of these domains are in metallopeptidases, whereas others are in prolyl 4-hydroxylases, tyrosinases, peroxidases, oxidoreductases, or proteins containing epidermal growth factor-like domains, thrombospondin-type repeats, or trypsin-like serine protease domains.[14][15][16][17][18] The only human proteins containing ShK-like domains are MMP-23 (matrix metalloprotease 23) and MFAP-2 (microfibril-associated glycoprotein 2).[14][15][16][17][18]
Channel targets
File:ShK toxin Fig 5.tiff The ShK peptide blocks potassium (K+) ion channels Kv1.1, Kv1.3, Kv1.6, Kv3.2 and KCa3.1 with nanomolar to picomolar potency, and has no effect on the HERG (Kv11.1) cardiac potassium channel.[19][20] The neuronal Kv1.1 channel and the T lymphocyte Kv1.3 channel are most potently inhibited by ShK.[8]
Binding configuration in K+ channels
ShK and its analogues are blockers of the channel pore. They bind to all four subunits in the K+ channel tetramer by interacting with the shallow 'vestibule' at the outer entrance to the channel pore.[5][8][9][12][21][22][19] These peptides are anchored in the external vestibule by two key interactions. The first is Lys22, which protrudes into and occludes the channel's pore like a "cork in a bottle" and blocks the passage of potassium ions through the channel pore.[8][23][22][19] The second is the neighboring Tyr23, which together with Lys22 forms a “functional dyad” required for channel block.[8][9][22][23][19] Many K+ channel-blocking peptides contain such a dyad of a lysine and a neighboring aromatic or aliphatic residue.[19][20] Some K+ channel-blocking peptides lack the functional dyad, but even in these peptides a lysine physically blocks the channel, regardless of the position of the lysine in the peptide sequence.[24] Additional interactions anchor ShK and its analogues in the external vestibule and contribute to potency and selectivity.[8][9][22][23][19] For example, Arg11 and Arg29 in ShK interact with two Asp386 residues in adjacent subunits in the mouse Kv1.3 external vestibule (corresponds to Asp433 in human Kv1.3).[8][9][22][23][19]
| Channel | ShK(IC50) | ShK-186
(IC50) | ShK-192
(IC50) | ShK-EWSS
(IC50) | ShK-F6CA (IC50) | ShK-198 (IC50) | MMP-23 ShK domain (IC50) |
| Kv1.1 | 16-28 pM | 7 nM | 22 nM | 5.4 nM | 4 nM | 159 pM | 49 μM |
| Kv1.2 | 10 nM | 48 nM | ND | >100 nM | >100 nM | ND | >100 μM |
| Kv1.3 | 10-16 pM | 70 pM | 140 pM | 34 pM | 48 pM | 41 pM | 2.8 μM |
| Kv1.6 | 200 pM | 18 nM | 10.6 nM | ND | ND | ND | 400 nM |
| Kv3.2 | 5 nM | 20 nM | 4.2 nM | ND | ND | ND | 49 μM |
| KCa3.1 | 30 nM | 115 nM | >100 nM | >100 nM | ND | ND | >100 μM |
Analogues that block the Kv1.3 channel
Several ShK analogues have been generated to enhance specificity for the Kv1.3 channel over the neuronal Kv1.1 channel and other closely related channels.
- ShK-Dap22: This was the first analogue that showed some degree of specificity for Kv1.3. The pore-occluding lysine22 of ShK is replaced by diaminopropionic acid (Dap) in ShK-Dap22.[8][22][25] Dap is a non-natural lysine analogue with a shorter side chain length (2.5 Å from Cα) than lysine (6.3 Å).[26] Dap22 interacts with residues further out in the external vestibule in contrast to lysine22, which interacts with the channel's selectivity filter.[22] As a consequence, the orientations of ShK and ShK-Dap22 in the external vestibule are significantly different.[22] ShK-Dap22 exhibits >20-fold selectivity for Kv1.3 over closely related channels in whole-cell patch clamp experiments,[8] but in equilibrium binding assays it binds Kv1.1-Kv1.2 heterotetramers with almost the same potency as ShK, which is not predicted from the study of homotetrameric Kv1.1 or Kv1.2 channels.[25]
- ShK-F6CA: Attaching a fluorescein to the N-terminus of the peptide via a hydrophilic AEEA linker (2-aminoethoxy-2-ethoxy acetic acid; mini-PEG) resulted in a peptide, ShK-F6CA (fluorescein-6-carboxyl), with 100-fold specificity for Kv1.3 over Kv1.1 and related channels.[27] Attachment of a tetramethylrhodamine or a biotin via the AEEA linker to ShK's N-terminus did not increase specificity for Kv1.3 over Kv1.1.[27] The enhanced specificity of ShK-F6CA might be explained by differences in charge: F6CA is negatively charged; tetramethylrhodamine is positively charged; and biotin is neutral.[27] Subsequent studies with other analogues suggest that the negatively charged F6CA likely interacts with residues on the turret of the Kv1.3 channel as shown for ShK-192 and ShK-EWSS.[9][12]
- ShK-170, ShK-186, ShK-192 and ShK-EWSS: Based on ShK-F6CA, additional analogues were made. Attaching a L-phosphotyrosine to the N-terminus of ShK via an AEEA linker resulted in a peptide, ShK-170, with 100-1000-fold specificity for Kv1.3 over related channels. ShK-186 (a.k.a. SL5; a.k.a. Dalazatide) is identical to ShK-170 except the C-terminal carboxyl is replaced by an amide. ShK-186 blocks Kv1.3 with an IC50 of 69 pM and exhibits the same specificity for Kv1.3 over closely related channels as ShK-170.[19] The L-phosphotyrosine of ShK-170 and ShK-186 rapidly gets dephosphorylated in vivo generating an analogue, ShK-198, with reduced specificity for Kv1.3.[5][28][29] To overcome this problem, ShK-192 and ShK-EWSS were developed. In ShK-192, the N-terminal L-phosphotyrosine is replaced by a non-hydrolyzable para-phosphonophenylalanine (Ppa), and Met21 is replaced by the non-natural amino acid norleucine to avoid methionine oxidation.[9][29] In ShK-EWSS, the AEEA linker and L-phosphotyrosine are replaced by the residues glutamic acid (E), tryptophan (W) and two serines (S).[12] Both ShK-192 and ShK-EWSS are highly specific for Kv1.3 over related channels.
- ShK-K18A: Docking and molecular dynamics simulations on Kv1.3 and Kv1.1 followed by umbrella sampling simulations, paved the way to the selective Kv1.3 inhibitor ShK-K18A.[30]
- ShK-related peptides in parasitic worms: AcK1, a 51-residue peptide from hookworms Ancylostoma caninum and Ancylostoma ceylanicum, and BmK1, the C-terminal domain of a metalloprotease from filarial worm Brugia malayi, adopt helical structures closely resembling ShK.[31] AcK1 and BmK1 block Kv1.3 channels at nanomolar-micromolar concentrations, and they suppress rat effector memory T cells without affecting naïve and central memory T cell subsets.[31] Further, they suppress IFN-g production by human T cells and they inhibit the Delayed-type hypersensitivity response caused by skin-homing effector memory T cells.[31] Teladorsagia circumcincta is an economically important parasite that infects sheep and goats. TcK6, a 90-residue protein with a C-terminal ShK-related domain, is upregulated during the mucosal dwelling larval stage of this parasite.[32] TcK6 causes modest suppression of thapsigargin-triggered IFN-g production by sheep T cells, suggesting that the parasite use this protein for immune evasion by modulating mucosal T cells.[32]
Extending circulating half-life
Due to their low molecular mass, ShK and its analogues are prone to rapid renal elimination. In rats, the half-life is ~6 min for ShK-186 and ~11 min for ShK-198, with a clearance rate of ~950 ml/kg·min.[28] In monkeys, the half-life is ~12 min for ShK-186 and ~46 min for ShK-198, with a clearance rate of ~80 ml/kg·min.[28]
PEGylation of ShK: Conjugation of polyethylene glycol (PEG) to ShK[Q16K], an ShK analogue, increased its molecular mass and thereby reduced renal clearance and extended plasma half-life to 15 h in mice and 64 h in cynomolgus monkeys.[11] PEGylation can also decrease immunogenicity and protect a peptide from proteolysis and non-specific adsorption to inert surfaces. PEGylated ShK[Q16K] prevented adoptive-transfer experimental autoimmune encephalomyelitis in rats, a model for multiple sclerosis.[11]
- Conjugation of ShK to larger proteins: The circulating half-life of peptides can be prolonged by coupling them to larger proteins or protein domains.[19][33][34] By screening a combinatorial ShK peptide library, novel analogues were identified, which when fused to the C-termini of IgG1-Fc retained picomolar potency, effectively suppressed in vivo delayed type hypersensitivity and exhibited a prolonged circulating half-life.[35]
- Prolonged effects despite rapid plasma clearance: SPECT/CT imaging studies with a 111In-DOTA-conjugate of ShK-186 in rats and squirrel monkeys revealed a slow release from the injection site and blood levels above the channel blocking dose for 2 and 7 days, respectively.[28] Studies on human peripheral blood T cells showed that a brief exposure to ShK-186 was sufficient to suppress cytokine responses.[28] These findings suggest that ShK-186, despite its short circulating half-life, may have a prolonged therapeutic effect. In rats, the peptide is effective in treating disease in animal models of autoimmune diseases when administered once a day to once in 3 days.[28] In humans, subcutaneous injections twice a week are sufficient to ameliorate disease in patients with plaque psoriasis.[7]
Peptide delivery
The low molecular mass of ShK and its analogues, combined with their high isoelectric points, makes it unlikely that these peptides will be absorbed from the stomach or intestine following oral administration. Sub-lingual delivery is a possibility. A fluorescent ShK analogue was absorbed into the blood stream at pharmacological concentrations following sublingual administration with a mucoadhesive chitosan-based gel, with or without the penetration enhancer cetrimide.[36] Delivery of the peptide as an aerosol through the lung, or across the skin, or as eye drops are also possibilities.[37][38][39]
Modulation of T cell function
During T cell-activation, calcium enters lymphocytes through store-operated CRAC channels (calcium release activated channel) formed as a complex of Orai and Stim proteins.[40][41] The rise in intracellular calcium initiates a signaling cascade culminating in cytokine production and proliferation.[40][41] The Kv1.3 K+ channel and the calcium-activated KCa3.1 K+ channel in T cells promote calcium entry into the cytoplasm through CRAC by providing a counterbalancing cation efflux.[19][40][41] Blockade of Kv1.3 depolarizes the membrane potential of T cells, suppresses calcium signaling and IL-2 production, but not IL2-receptor expression.[42][43][44][45][46] Kv1.3 blockers have no effect on activation pathways that are independent of a rise in intracellular calcium (e.g. anti-CD28, IL-2).[42][43] Expression of the Kv1.3 and KCa3.1 channels varies during T cell activation and differentiation into memory T cells.[19][40][41][47][48] When naïve T cells and central memory T cells (TCM) are activated they upregulate KCa3.1 expression to ~500 per cell without significant change in Kv1.3 numbers.[19][40][41][47][48] In contrast, when terminally differentiated effector memory subsets (TEM, TEMRA [T effector memory re-expressing CD45RA]) are activated, they upregulate Kv1.3 to 1500 per cell without changes in KCa3.1.[19][40][41][47][48] The Kv1.3 channel number increases and the KCa3.1 channel number decreases as T cells are chronically activated.[40][41][47][48][49] As a result of this differential expression, blockers of KCa3.1 channels preferentially suppress the function of naïve and TCM cells, while ShK and its analogues that selectively inhibit Kv1.3 channels preferentially suppress the function of chronically activated effector memory T cells (TEM, TEMRA).[19][40][41][47][48]
Of special interest are the large number of ShK analogues developed at Amgen that suppressed interleukin-2 and interferon gamma production by T cells.[11] This inhibitory effect of Kv1.3 blockers is partial and stimulation strength dependent, with reduced inhibitory efficacy on T cells under strengthened anti-CD3/CD28 stimulation.[50] Chronically activated CD28null effector memory T cells are implicated in autoimmune diseases (e.g. lupus, Crohn's disease, rheumatoid arthritis, multiple sclerosis).[51][52][53]
Blockade of Kv1.3 channels in these chronically activated T cells suppresses calcium signaling, cytokine production (interferon gamma, interleukin-2, interleukin 17), and cell proliferation.[6][19][29][30][40][41][47][48] Effector memory T cells that are CD28+ are refractory to suppression by Kv1.3 blockers when they are co-stimulated by anti-CD3 and anti-CD28 antibodies, but are sensitive to suppression when stimulated by anti-CD3 antibodies alone.[48] In vivo, ShK-186 paralyzes effector-memory T cells at the site of an inflammatory delayed type hypersensitivity response and prevents these T cells from activating in the inflamed tissue.[54] In contrast, ShK-186 does not affect the homing and motility of naive and TCM cells to and within lymph nodes, most likely because these cells express the KCa3.1 channel and are therefore protected from the effect of Kv1.3 blockade.[54]
Effects on microglia
Kv1.3 plays an important role in microglial activation.[55][56][57][58] ShK-223, an analogue of ShK-186, decreased lipopolysaccharide (LPS) induced focal adhesion formation by microglia, reversed LPS-induced inhibition of microglial migration, and inhibited LPS-induced upregulation of EH domain containing protein 1 (EHD1), a protein involved in microglia trafficking.[59] Increased Kv1.3 expression was reported in microglia in Alzheimer plaques.[60] Kv1.3 inhibitors may have use in the management of Alzheimer's disease, as reported in a proof-of-concept study in which a small molecule Kv1.3 blocker (PAP-1) alleviated Alzheimer's disease-like characteristics in a mouse model of AD.[61]
Efficacy of analogues in animal models of human diseases
Experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis
ShK, ShK-Dap22, ShK-170 and PEGylated ShK-Q16K prevent adoptive-transfer EAE in Lewis rats, a model of multiple sclerosis.[5][11] Since multiple sclerosis is a relapsing-remitting disease, ShK-186 and ShK-192 were evaluated in a relapsing-remitting EAE model in DA (Dark Agouti) rats. Both prevented and treated disease when administered once a day to once in three days.[54][28][29] Thus, Kv1.3 inhibitors are effective in treating disease in rat models of multiple sclerosis when administered alone,[11][28][62][63] and therapeutic effectiveness does not appear to be compromised by compensatory over-expression of KCa3.1 channels.[49][64]
Pristane-induced arthritis (PIA), a model for rheumatoid arthritis
ShK-186 was effective in treating PIA when administered every day or on alternate days.[5][6][54][28] A scorpion toxin inhibitor of KV1.3 was also effective in this model.[65] In both these studies, blockade of Kv1.3 alone was sufficient to ameliorate disease and simultaneous blockade of KCa3.1 was not necessary as has been suggested.[49][64]
Rat models of atopic dermatitis
Most infiltrating T-cells in skin lesions from patients with moderate-to-severe atopic dermatitis (AD) express high levels of Kv1.3, suggesting that inhibitors of Kv1.3 may be effective in treating AD.[66] Ovalbumin-induced delayed type hypersensitivity and oxazolone-induced dermatitis are considered to be models of atopic dermatitis.[66][67][68][69][70] ShK, ShK-170, ShK-186, ShK-192 and ShK-IgG-Fc were all effective in the ovalbumin-induced delayed type hypersensitivity model,[5][6][9][54][28][29][35][66][69][70][71][72][73][74][75] while a topical formulation of ShK-198 was effective in treating oxazolone-induced dermatitis.[66] Even where compensation by KCa3.1 channels was reported to over-ride KV1.3 block, ShK administered alone suppressed delayed type hypersensitivity significantly in 2 of 3 studies, albeit modestly.[49]
Psoriasis
Psoriasis is a severe autoimmune disease of the skin that afflicts many people worldwide. Despite the success of recent biologics in ameliorating disease, there is still a search for safe and effective drugs for psoriasis. KV1.3 inhibitors (ShK, PAP-1) have been reported to treat disease in psoriasiform (psoriasis-like) SCID (severe combined immunodeficiency) mouse model.[76] In a Phase 1b placebo-controlled clinical study in patients with plaque psoriasis, ShK-186 administered twice a week (30 or 60 mg/dose/patient) by subcutaneous injection caused improvements with a statistically significant reduction in their PASI (Psoriasis Area and Severity Index) score between baseline and day 32.[7] These patients also exhibited reduced plasma levels of multiple inflammation markers and decreased expression of T cell activation markers on peripheral blood memory T cells.[7]
Diet-induced obesity and fatty liver disease
Obesity and diabetes are major healthcare problems globally. There is need for safe drugs for these metabolic diseases. In a mouse model of diet-induced obesity, ShK-186 counteracted the negative effects of increased caloric intake. It reduced weight gain, adiposity, and fatty liver; decreased blood levels of cholesterol, sugar, HbA1c, insulin, and leptin; and enhanced peripheral insulin sensitivity.[77] Genetic deletion of the Kv1.3 gene has the same effect, indicating that ShK-186's effect is due to Kv1.3 blockade.[78][77][79][80] At least two mechanisms contribute to ShK-186's therapeutic benefits. The high calorie diet induced Kv1.3 expression in brown fat tissues.[77] By blocking Kv1.3, ShK-186 doubled glucose uptake and increased β-oxidation of fatty acids, glycolysis, fatty acid synthesis and uncoupling protein 1 expression by brown fat.[77] As a consequence of brown fat activation, oxygen consumption and energy expenditure were augmented.[77] The obesity diet also induced Kv1.3 expression in the liver, and ShK-186 caused profound alterations in energy and lipid metabolism in the liver. ShK, its analogues or other Kv1.3 blockers may have use in controlling the negative consequences of high calorie diets.
Arousal and anesthesia
The mechanisms of general anesthesia involve multiple molecular targets and pathways that are not completely understood.[81] Sevoflurane is a common anesthetic used to induce general anesthesia during surgery.[81] Rats continually exposed to sevoflurane lose their righting reflex as an index of loss of consciousness. In these rats, microinfusion of ShK into the central medial thalamic nucleus (CMT) reversed sevoflurane-induced anesthesia in rodents.[81] ShK-treated rats righted themselves fully (restored consciousness) despite being continually exposed to sevoflurane.[81] ShK-microinfusion into neighboring regions of the brain did not have this effect.[81] Sevoflurane enhanced potassium currents in the CMT, while ShK and ShK-186 countered this effect.[81] These studies suggest that ShK-sensitive K+ channels in the CMT are important for suppressing arousal during anesthesia.
Preventing brain damage following therapeutic brain radiation
Brain radiation is used to treat tumors of the head, neck, and brain, but this treatment carries a significant risk of neurologic injury. Injury is, in part, due to the activation of microglia and microglia-mediated damage of neurons. Neuroprotective therapies for radiation-induced brain injury are still limited. In a mouse model of brain radiation, ShK-170 reversed neurological deficits, and protected neurons from radiation-induced brain injury by suppressing microglia.[82]
Toxicity of ShK and its analogues
ShK and ShK-Dap22
ShK peptide has a low toxicity profile in mice. ShK is effective in treating autoimmune diseases at 10 to 100 mg/kg bodyweight. It has a median paralytic dose of approximately 25 mg/kg bodyweight (250-2500 higher than the pharmacological dose). In rats the therapeutic safety index is greater than 75-fold. ShK-Dap22 displayed a lower toxicity profile.[8] A 1.0 mg dose did not induce any hyperactivity, seizures or mortality in rats. The median paralytic dose for ShK-Dap22 is about 200 mg/kg bodyweight (2000-20000 higher than pharmacological dose).[8] PEGylated ShK[Q16K] showed no adverse toxicity in monkeys over a period of several months.[11]
ShK-186/Dalazatide
ShK-186 also displays a low toxicity profile in rats. Daily administration of ShK-170 or ShK-186 (100 µg/kg/day) by subcutaneous injection over 4 weeks in rats does not induce any changes in blood counts, blood chemistry or histopathology.[5][6][28] By virtue of suppressing only TEM and TEMRA cells, ShK-186 did not compromise protective immune responses to influenza virus and chlamydial infection in rats, most likely because naïve and TCM cells unaffected by Kv1.3 blockade mounted effective immune responses.[54] ShK-186 is poorly immunogenic and did not elicit anti-ShK antibodies in rats repeatedly administered the peptide.[6] This is possibly because the peptide's disulfide-bonded structure hinders processing and antigen presentation by antigen-presenting cells. ShK-186 also shares sequence and structural similarity to a ShK-like domain in matrix metalloprotease 23,[14][15][16][17][18] which may cause the immune system to assume it is a normal protein in the body. ShK-186 was safe in non-human primates. In Phase 1a and 1b trials in healthy human volunteers, ShK-186 was well tolerated, no grade 3 or 4 adverse effects or laboratory abnormalities were noted, and the predicted range of drug exposures were achieved.[7] The most common adverse events were temporary mild (Grade 1) hypoesthesia and paresthesia involving the hands, feet, or perioral area. Mild muscle spasms, sensitivity of teeth, and injection site pain were also observed.[7]
Functions of ShK-like proteins
MMP-23
MMP-23 belongs to the family of zinc- and calcium-dependent matrix metalloproteases. It is anchored in the cell membrane by an N-terminal prodomain, and it contains three extracellular domains: catalytic metalloprotease domain, ShK domain and immunoglobulin-like cell adhesion molecule (Ig-CaM) domain.[14][15][16][17][18] The prodomain traps the voltage-gated potassium channel KV1.3, but not the closely related KV1.2 channel, in the endoplasmic reticulum.[14][15][16][17] Studies with chimeras suggest that the prodomain interacts with the KV1.3 region from the S5 transmembrane segment to the C terminus.[14][15][16][17] NMR studies of the prodomain reveal a single trans-membrane alpha-helix, joined by a short linker to a juxta-membrane alpha-helix, which is associated with the surface of the membrane.[14][15][16][17] The prodomain shares topological similarity with proteins (KCNE1, KCNE2, KCNE4) known to trap potassium channels in the secretory pathway, suggesting a shared mechanism of channel regulation.[14][15][16][17] MMP-23's catalytic domain displays structural homology with catalytic domains in other metalloproteases, and likely functions as an endopeptidase. MMP-23's ShK domain lies immediately after the catalytic domain and is connected to the IgCAM domain by a short proline-rich linker. It shares phylogenetic relatedness to sea anemone toxins and ICR-CRISP domains, being most similar to the BgK toxin from sea anemone Bunodosoma granulifera.[14][15][16][17] This ShK domain blocks voltage-gated potassium channels (KV1.6 > KV1.3 > KV1.1 = KV3.2 > Kv1.4, in decreasing potency) in the nanomolar to low micromolar range.[14] KV1.3 is required for sustaining calcium signaling during activation of human T cells.[19][40][47] By trapping KV1.3 in the endoplasmic reticulum via the prodomain, and by blocking the KV1.3 channel with the ShK domain, MMP-23 may serve as an immune checkpoint to reduce excessive T cell activation during an immune response. In support, increased expression of MMP-23 in melanoma cancer cells decreases tumor-infiltrating lymphocytes, and is associated with cancer recurrence and shorter periods of progression-free survival.[17] However, in melanomas, expression of MMP-23 does not correlate with Kv1.3 expression, suggesting that MMP-23's deleterious effect in melanomas may not be connected with its Kv1.3 channel-modulating function.[17] MMP-23's C-terminal IgCAM domain shares sequence similarity with IgCAM domains in proteins known to mediate protein-protein and protein-lipid interactions (e.g. CDON, human Brother of CDO, ROBO1-4, hemicentin, NCAM1 and NCAM2).[14][15][16][17] In summary, the four domains of MMP-23 may work synergistically to modulate immune responses in vivo.
Mab7
In male Caenorhabditis elegans worms, the absence of a protein called Mab7 (Q95Q39) results in malformed sensory rays that are required for mating.[63] Introduction of Mab7 into these male worms restores normal development of normal sensory rays.[63] Introduction of Mab7 proteins lacking the ShK domain does not correct the defect of sensory rays, suggesting a role for the ShK-domain of Mab7 in sensory ray development.[63]
HMP2 and PMP1
HMP2 and PMP-1 are astacin metalloproteinases from the Cnidarian Hydra vulgaris and the jellyfish Podocoryne carnea that contain ShK-like domains at their C-termini.[83][17] Both these ShK-domains contain the critical pore-occluding lysine required for K+ channel block.[14] HMP2 plays a critical role in foot regeneration of Hydra,[83] while PMP-1 is found in the feeding organ of the jelly fish and the ShK-domain may paralyze prey after they are ingested.[17]
CRISPs
More distantly related are Cysteine-rich secretory proteins (CRISPs), which contain a ShK-like 'Cystine-rich domain' as well as a larger CAP-like 'Pathogenesis related 1' domain.[84] These proteins are involved in mammalian reproduction[85] as well as in the venoms of some snakes.[86] In both cases, the mechanism is believed to involve inhibition of ion channel activity.[85]
References
- ↑ "Potassium channel blockade by the sea anemone toxin ShK for the treatment of multiple sclerosis and other autoimmune diseases". Current Medicinal Chemistry 11 (23): 3041–52. December 2004. doi:10.2174/0929867043363947. PMID 15578998.
- ↑ "Characterization of a potassium channel toxin from the Caribbean Sea anemone Stichodactyla helianthus". Toxicon 33 (5): 603–13. May 1995. doi:10.1016/0041-0101(95)00013-C. PMID 7660365.
- ↑ "Chemical synthesis and characterization of ShK toxin: a potent potassium channel inhibitor from a sea anemone". International Journal of Peptide and Protein Research 46 (5): 354–8. November 1995. doi:10.1111/j.1399-3011.1995.tb01068.x. PMID 8567178.
- ↑ 4.0 4.1 4.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedTudor_1996 - ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases". Molecular Pharmacology 67 (4): 1369–81. April 2005. doi:10.1124/mol.104.008193. PMID 15665253.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 "Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases". Proceedings of the National Academy of Sciences of the United States of America 103 (46): 17414–9. November 2006. doi:10.1073/pnas.0605136103. PMID 17088564. Bibcode: 2006PNAS..10317414B.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial". PLOS ONE 12 (7): e0180762. July 2017. doi:10.1371/journal.pone.0180762. PMID 28723914. Bibcode: 2017PLoSO..1280762T.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 "ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide". The Journal of Biological Chemistry 273 (49): 32697–707. December 1998. doi:10.1074/jbc.273.49.32697. PMID 9830012.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 "Engineering a stable and selective peptide blocker of the Kv1.3 channel in T lymphocytes". Molecular Pharmacology 75 (4): 762–73. April 2009. doi:10.1124/mol.108.052704. PMID 19122005.
- ↑ "A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3". FEBS Letters 586 (22): 3996–4001. November 2012. doi:10.1016/j.febslet.2012.09.038. PMID 23063513.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 "Pharmaceutical Optimization of Peptide Toxins for Ion Channel Targets: Potent, Selective, and Long-Lived Antagonists of Kv1.3". Journal of Medicinal Chemistry 58 (17): 6784–802. September 2015. doi:10.1021/acs.jmedchem.5b00495. PMID 26288216.
- ↑ 12.0 12.1 12.2 12.3 "N-Terminally extended analogues of the K⁺ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3". The FEBS Journal 282 (12): 2247–59. June 2015. doi:10.1111/febs.13294. PMID 25864722.
- ↑ "SMART: ShKT domain annotation". http://smart.embl-heidelberg.de/smart/do_annotation.pl?ACC=SM00254.
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 "Potassium channel modulation by a toxin domain in matrix metalloprotease 23". The Journal of Biological Chemistry 285 (12): 9124–36. March 2010. doi:10.1074/jbc.M109.071266. PMID 19965868.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 "Intracellular trafficking of the KV1.3 potassium channel is regulated by the prodomain of a matrix metalloprotease". The Journal of Biological Chemistry 288 (9): 6451–64. March 2013. doi:10.1074/jbc.M112.421495. PMID 23300077.
- ↑ 16.00 16.01 16.02 16.03 16.04 16.05 16.06 16.07 16.08 16.09 16.10 "Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking". Cellular and Molecular Life Sciences 71 (7): 1191–210. April 2014. doi:10.1007/s00018-013-1431-0. PMID 23912897.
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 17.12 17.13 17.14 "Melanoma expression of matrix metalloproteinase-23 is associated with blunted tumor immunity and poor responses to immunotherapy". Journal of Translational Medicine 12: 342. December 2014. doi:10.1186/s12967-014-0342-7. PMID 25491880.
- ↑ 18.0 18.1 18.2 18.3 18.4 "A toxin homology domain in an astacin-like metalloproteinase of the jellyfish Podocoryne carnea with a dual role in digestion and development". Development Genes and Evolution 208 (5): 259–66. July 1998. doi:10.1007/s004270050180. PMID 9683741.
- ↑ 19.00 19.01 19.02 19.03 19.04 19.05 19.06 19.07 19.08 19.09 19.10 19.11 19.12 19.13 19.14 19.15 "v1.3 channels in T cells as therapeutics for autoimmune disease". Current Opinion in Chemical Biology 38: 97–107. June 2017. doi:10.1016/j.cbpa.2017.02.015. PMID 28412597.
- ↑ 20.0 20.1 "A variable residue in the pore of Kv1 channels is critical for the high affinity of blockers from sea anemones and scorpions". The Journal of Biological Chemistry 280 (29): 27093–102. July 2005. doi:10.1074/jbc.M413626200. PMID 15890656.
- ↑ "An essential binding surface for ShK toxin interaction with rat brain potassium channels". Biochemistry 35 (51): 16407–11. December 1996. doi:10.1021/bi962463g. PMID 8987971.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 "Mutating a critical lysine in ShK toxin alters its binding configuration in the pore-vestibule region of the voltage-gated potassium channel, Kv1.3". Biochemistry 41 (40): 11963–71. October 2002. doi:10.1021/bi026400b. PMID 12356296.
- ↑ 23.0 23.1 23.2 23.3 "Structural conservation of the pores of calcium-activated and voltage-gated potassium channels determined by a sea anemone toxin". The Journal of Biological Chemistry 274 (31): 21885–92. July 1999. doi:10.1074/jbc.274.31.21885. PMID 10419508.
- ↑ "Looking over toxin-K(+) channel interactions. Clues from the structural and functional characterization of α-KTx toxin Tc32, a Kv1.3 channel blocker". Biochemistry 51 (9): 1885–94. March 2012. doi:10.1021/bi201713z. PMID 22332965.
- ↑ 25.0 25.1 "Substitution of a single residue in Stichodactyla helianthus peptide, ShK-Dap22, reveals a novel pharmacological profile". Biochemistry 42 (46): 13698–707. November 2003. doi:10.1021/bi035209e. PMID 14622016.
- ↑ "The signature sequence of voltage-gated potassium channels projects into the external vestibule". The Journal of Biological Chemistry 271 (49): 31013–6. December 1996. doi:10.1074/jbc.271.49.31013. PMID 8940091.
- ↑ 27.0 27.1 27.2 "A novel fluorescent toxin to detect and investigate Kv1.3 channel up-regulation in chronically activated T lymphocytes". The Journal of Biological Chemistry 278 (11): 9928–37. March 2003. doi:10.1074/jbc.M212868200. PMID 12511563.
- ↑ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 28.10 "Durable pharmacological responses from the peptide ShK-186, a specific Kv1.3 channel inhibitor that suppresses T cell mediators of autoimmune disease". The Journal of Pharmacology and Experimental Therapeutics 342 (3): 642–53. September 2012. doi:10.1124/jpet.112.191890. PMID 22637724.
- ↑ 29.0 29.1 29.2 29.3 29.4 "Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases". Toxicon 59 (4): 529–46. March 2012. doi:10.1016/j.toxicon.2011.07.016. PMID 21867724.
- ↑ 30.0 30.1 "A potent and selective peptide blocker of the Kv1.3 channel: prediction from free-energy simulations and experimental confirmation". PLOS ONE 8 (11): e78712. November 2013. doi:10.1371/journal.pone.0078712. PMID 24244345. Bibcode: 2013PLoSO...878712R.
- ↑ 31.0 31.1 31.2 "Kv1.3 channel-blocking immunomodulatory peptides from parasitic worms: implications for autoimmune diseases". FASEB Journal 28 (9): 3952–64. September 2014. doi:10.1096/fj.14-251967. PMID 24891519.
- ↑ 32.0 32.1 "Niche-specific gene expression in a parasitic nematode; increased expression of immunomodulators in Teladorsagia circumcincta larvae derived from host mucosa". Scientific Reports 7 (1): 7214. August 2017. doi:10.1038/s41598-017-07092-0. PMID 28775251. Bibcode: 2017NatSR...7.7214M.
- ↑ "Targeting the ion channel Kv1.3 with scorpion venom peptides engineered for potency, selectivity, and half-life". The Journal of Biological Chemistry 289 (33): 22704–14. August 2014. doi:10.1074/jbc.M114.568642. PMID 24939846.
- ↑ "Rational design of a Kv1.3 channel-blocking antibody as a selective immunosuppressant". Proceedings of the National Academy of Sciences of the United States of America 113 (41): 11501–11506. October 2016. doi:10.1073/pnas.1612803113. PMID 27663736. Bibcode: 2016PNAS..11311501W.
- ↑ 35.0 35.1 "Autocrine-Based Selection of Drugs That Target Ion Channels from Combinatorial Venom Peptide Libraries". Angewandte Chemie 55 (32): 9306–10. August 2016. doi:10.1002/anie.201603052. PMID 27197631.
- ↑ "Buccal mucosal delivery of a potent peptide leads to therapeutically-relevant plasma concentrations for the treatment of autoimmune diseases". Journal of Controlled Release 199: 37–44. February 2015. doi:10.1016/j.jconrel.2014.12.001. PMID 25482338.
- ↑ "Pulmonary Delivery of the Kv1.3-Blocking Peptide HsTX1[R14A] for the Treatment of Autoimmune Diseases". Journal of Pharmaceutical Sciences 105 (2): 650–656. February 2016. doi:10.1016/j.xphs.2015.10.025. PMID 26869426.
- ↑ Iadonato & Munoz, "Ophthalmic uses of toxin-based therapeutic peptides and pharmaceutical compositions thereof", WO patent 2015013330
- ↑ Iadonato; Tarcha & Lustig, "Topical applications of kv1.3 channel blocking peptides to treat skin inflammation", WO patent 2016112208
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 40.8 40.9 "The functional network of ion channels in T lymphocytes". Immunological Reviews 231 (1): 59–87. September 2009. doi:10.1111/j.1600-065x.2009.00816.x. PMID 19754890.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 41.8 "Ion channels in innate and adaptive immunity". Annual Review of Immunology 33 (1): 291–353. March 2015. doi:10.1146/annurev-immunol-032414-112212. PMID 25861976.
- ↑ 42.0 42.1 "Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation". The Journal of Experimental Medicine 177 (3): 637–45. March 1993. doi:10.1084/jem.177.3.637. PMID 7679705.
- ↑ 43.0 43.1 "Voltage-gated potassium channels are required for human T lymphocyte activation". The Journal of Experimental Medicine 160 (2): 369–85. August 1984. doi:10.1084/jem.160.2.369. PMID 6088661.
- ↑ "Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin". Proceedings of the National Academy of Sciences of the United States of America 89 (21): 10094–8. November 1992. doi:10.1073/pnas.89.21.10094. PMID 1279670. Bibcode: 1992PNAS...8910094L.
- ↑ "Stable expression of human Kv1.3 potassium channels resets the resting membrane potential of cultured mammalian cells". Receptors & Channels 3 (4): 273–81. 1995. PMID 8834000.
- ↑ "Voltage-gated and Ca(2+)-activated K+ channels in intact human T lymphocytes. Noninvasive measurements of membrane currents, membrane potential, and intracellular calcium". The Journal of General Physiology 105 (6): 765–94. June 1995. doi:10.1085/jgp.105.6.765. PMID 7561743.
- ↑ 47.0 47.1 47.2 47.3 47.4 47.5 47.6 "The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS". The Journal of Clinical Investigation 111 (11): 1703–13. June 2003. doi:10.1172/jci16921. PMID 12782673.
- ↑ 48.0 48.1 48.2 48.3 48.4 48.5 48.6 "Characterization of the functional properties of the voltage-gated potassium channel Kv1.3 in human CD4+ T lymphocytes". Journal of Immunology 179 (7): 4563–70. October 2007. doi:10.4049/jimmunol.179.7.4563. PMID 17878353.
- ↑ 49.0 49.1 49.2 49.3 "Potassium channels Kv1.3 and KCa3.1 cooperatively and compensatorily regulate antigen-specific memory T cell functions". Nature Communications 8: 14644. March 2017. doi:10.1038/ncomms14644. PMID 28248292. Bibcode: 2017NatCo...814644C.
- ↑ "T Cell Subset and Stimulation Strength-Dependent Modulation of T Cell Activation by Kv1.3 Blockers". PLOS ONE 12 (1): e0170102. 2017-01-20. doi:10.1371/journal.pone.0170102. PMID 28107393. Bibcode: 2017PLoSO..1270102F.
- ↑ "Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis". Journal of Immunology 165 (2): 1138–45. July 2000. doi:10.4049/jimmunol.165.2.1138. PMID 10878393.
- ↑ "CD4+CD28- costimulation-independent T cells in multiple sclerosis". The Journal of Clinical Investigation 108 (8): 1185–94. October 2001. doi:10.1172/jci12516. PMID 11602626.
- ↑ "Active Crohn's disease patients show a distinctive expansion of circulating memory CD4+CD45RO+CD28null T cells". Journal of Clinical Immunology 24 (2): 185–96. March 2004. doi:10.1023/B:JOCI.0000019784.20191.7f. PMID 15024186.
- ↑ 54.0 54.1 54.2 54.3 54.4 54.5 "Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block". Immunity 29 (4): 602–14. October 2008. doi:10.1016/j.immuni.2008.07.015. PMID 18835197.
- ↑ "K+ channels and the microglial respiratory burst". American Journal of Physiology. Cell Physiology 280 (4): C796–806. April 2001. doi:10.1152/ajpcell.2001.280.4.C796. PMID 11245596.
- ↑ "Microglia Kv1.3 channels contribute to their ability to kill neurons". The Journal of Neuroscience 25 (31): 7139–49. August 2005. doi:10.1523/jneurosci.1251-05.2005. PMID 16079396.
- ↑ "Differential Kv1.3, KCa3.1, and Kir2.1 expression in "classically" and "alternatively" activated microglia". Glia 65 (1): 106–121. January 2017. doi:10.1002/glia.23078. PMID 27696527.
- ↑ "Potassium channel expression and function in microglia: Plasticity and possible species variations". Channels 11 (4): 305–315. July 2017. doi:10.1080/19336950.2017.1300738. PMID 28277939.
- ↑ "A systems pharmacology-based approach to identify novel Kv1.3 channel-dependent mechanisms in microglial activation". Journal of Neuroinflammation 14 (1): 128. June 2017. doi:10.1186/s12974-017-0906-6. PMID 28651603.
- ↑ "Potassium channel Kv1.3 is highly expressed by microglia in human Alzheimer's disease". Journal of Alzheimer's Disease 44 (3): 797–808. 2015-01-01. doi:10.3233/jad-141704. PMID 25362031.
- ↑ "Kv1.3 inhibition as a potential microglia-targeted therapy for Alzheimer's disease: preclinical proof of concept". Brain 141 (2): 596–612. February 2018. doi:10.1093/brain/awx346. PMID 29272333.
- ↑ "Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis". Proceedings of the National Academy of Sciences of the United States of America 98 (24): 13942–7. November 2001. doi:10.1073/pnas.241497298. PMID 11717451. Bibcode: 2001PNAS...9813942B.
- ↑ 63.0 63.1 63.2 63.3 "mab-7 encodes a novel transmembrane protein that orchestrates sensory ray morphogenesis in C. elegans". Developmental Biology 312 (1): 353–66. December 2007. doi:10.1016/j.ydbio.2007.09.037. PMID 17959165. http://repository.ust.hk/ir/bitstream/1783.1-3295/1/Tsangetal20070908.pdf.
- ↑ 64.0 64.1 "Differential effects of membrane perturbants on voltage-activated sodium and calcium channels and calcium-dependent potassium channels". Biophysical Journal 45 (1): 132–4. January 1984. doi:10.1016/s0006-3495(84)84137-5. PMID 19431539. Bibcode: 1984BpJ....45..132H.
- ↑ "Prolonged immunomodulation in inflammatory arthritis using the selective Kv1.3 channel blocker HsTX1[R14A and its PEGylated analog"]. Clinical Immunology 180: 45–57. July 2017. doi:10.1016/j.clim.2017.03.014. PMID 28389388.
- ↑ 66.0 66.1 66.2 66.3 "695 Topical application of KPI-150, a potent inhibitor of Kv1.3 channels on effector memory T cells, demonstrates preclinical efficacy in atopic dermatitis model". Journal of Investigative Dermatology 137 (5): S119. 2017. doi:10.1016/j.jid.2017.02.718.
- ↑ "Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling". The Journal of Allergy and Clinical Immunology 139 (2): 562–571. February 2017. doi:10.1016/j.jaci.2016.08.029. PMID 27702671.
- ↑ "Translational Animal Models of Atopic Dermatitis for Preclinical Studies". The Yale Journal of Biology and Medicine 90 (3): 389–402. September 2017. PMID 28955179.
- ↑ 69.0 69.1 "Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis". The Journal of Investigative Dermatology 127 (6): 1419–29. June 2007. doi:10.1038/sj.jid.5700717. PMID 17273162.
- ↑ 70.0 70.1 "Kv1.3 blockers ameliorate allergic contact dermatitis by preferentially suppressing effector memory T cells in a rat model". Clinical and Experimental Dermatology 38 (8): 897–903. December 2013. doi:10.1111/ced.12097. PMID 24252082.
- ↑ "Correolide and derivatives are novel immunosuppressants blocking the lymphocyte Kv1.3 potassium channels". Cellular Immunology 197 (2): 99–107. November 1999. doi:10.1006/cimm.1999.1569. PMID 10607427.
- ↑ "Voltage-gated potassium channel (K(v) 1) autoantibodies in patients with chagasic gut dysmotility and distribution of K(v) 1 channels in human enteric neuromusculature (autoantibodies in GI dysmotility)". Neurogastroenterology and Motility 24 (8): 719–28, e344. August 2012. doi:10.1111/j.1365-2982.2012.01924.x. PMID 22591165.
- ↑ "Selective blocking of voltage-gated K+ channels improves experimental autoimmune encephalomyelitis and inhibits T cell activation". Journal of Immunology 166 (2): 936–44. January 2001. doi:10.4049/jimmunol.166.2.936. PMID 11145670.
- ↑ "Vm24, a natural immunosuppressive peptide, potently and selectively blocks Kv1.3 potassium channels of human T cells". Molecular Pharmacology 82 (3): 372–82. September 2012. doi:10.1124/mol.112.078006. PMID 22622363.
- ↑ "Kv1.3 in psoriatic disease: PAP-1, a small molecule inhibitor of Kv1.3 is effective in the SCID mouse psoriasis--xenograft model". Journal of Autoimmunity 55: 63–72. December 2014. doi:10.1016/j.jaut.2014.07.003. PMID 25175978.
- ↑ "The beneficial effect of blocking Kv1.3 in the psoriasiform SCID mouse model". The Journal of Investigative Dermatology 131 (1): 118–24. January 2011. doi:10.1038/jid.2010.245. PMID 20739949.
- ↑ 77.0 77.1 77.2 77.3 77.4 "Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance". Proceedings of the National Academy of Sciences of the United States of America 110 (24): E2239–48. June 2013. doi:10.1073/pnas.1221206110. PMID 23729813. Bibcode: 2013PNAS..110E2239U.
- ↑ "Kv1.3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice". International Journal of Obesity 32 (8): 1222–32. August 2008. doi:10.1038/ijo.2008.77. PMID 18542083.
- ↑ "The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight". Human Molecular Genetics 12 (5): 551–9. March 2003. doi:10.1093/hmg/ddg049. PMID 12588802.
- ↑ "The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity". Proceedings of the National Academy of Sciences of the United States of America 101 (9): 3112–7. March 2004. doi:10.1073/pnas.0308450100. PMID 14981264. Bibcode: 2004PNAS..101.3112X.
- ↑ 81.0 81.1 81.2 81.3 81.4 81.5 "Shaker-related potassium channels in the central medial nucleus of the thalamus are important molecular targets for arousal suppression by volatile general anesthetics". The Journal of Neuroscience 33 (41): 16310–22. October 2013. doi:10.1523/jneurosci.0344-13.2013. PMID 24107962.
- ↑ "Blockade of Kv1.3 channels ameliorates radiation-induced brain injury". Neuro-Oncology 16 (4): 528–39. April 2014. doi:10.1093/neuonc/not221. PMID 24305723.
- ↑ 83.0 83.1 "Identification and characterization of hydra metalloproteinase 2 (HMP2): a meprin-like astacin metalloproteinase that functions in foot morphogenesis". Development 127 (1): 129–41. January 2000. doi:10.1242/dev.127.1.129. PMID 10654607. http://dev.biologists.org/content/127/1/129.short.
- ↑ 84.0 84.1 "Crystal structure of the cysteine-rich secretory protein stecrisp reveals that the cysteine-rich domain has a K+ channel inhibitor-like fold". The Journal of Biological Chemistry 280 (13): 12405–12. April 2005. doi:10.1074/jbc.M413566200. PMID 15596436.
- ↑ 85.0 85.1 "The role of cysteine-rich secretory proteins in male fertility". Asian Journal of Andrology 13 (1): 111–7. January 2011. doi:10.1038/aja.2010.77. PMID 20972450.
- ↑ "Structure and function of snake venom cysteine-rich secretory proteins". Toxicon 44 (3): 227–31. September 2004. doi:10.1016/j.toxicon.2004.05.023. PMID 15302528.
External links
- Overview of all the structural information available in the PDB for UniProt: P29187 (Kappa-stichotoxin-She3a) at the PDBe-KB.
 |




