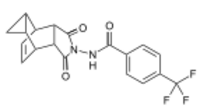
Chemistry:Tecovirimat
 | |
| Clinical data | |
|---|---|
| Trade names | Tpoxx |
| Other names | ST-246 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H15F3N2O3 |
| Molar mass | 376.335 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tecovirimat, sold under the brand name Tpoxx among others,[6] is an antiviral medication with activity against orthopoxviruses such as smallpox and mpox.[4][7][8] In 2018 it became the first antipoxviral drug approved in the United States.[9][10]
The drug works by blocking cellular transmission of the virus, thus preventing the disease.[11] It is an inhibitor of the orthopoxvirus VP37 envelope wrapping protein.[4]
Tecovirimat has been effective in laboratory testing; it has been shown to protect animals from mpox and rabbitpox and causes no serious side effects in humans.[6] Tecovirimat was first used for treatment in December 2018, after a laboratory-acquired vaccinia virus infection.[12]
Two million doses of tecovirimat are stockpiled in the US Strategic National Stockpile should an orthopoxvirus-based bioterror attack occur.[13][14] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[15]
Medical uses
In the United States, tecovirimat is indicated for the treatment of human smallpox disease.[4] In the European Union it is indicated for the treatment of smallpox, mpox, and cowpox.[5]
Mechanism of action
Tecovirimat inhibits the function of a major envelope protein required for the production of extracellular virus. The drug prevents the virus from leaving an infected cell, hindering the spread of the virus within the body.[16]
Chemistry
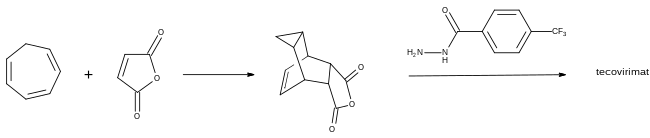
The first synthesis of tecovirimat was published in a patent filed by scientists at SIGA Technologies in 2004. It is made in two steps from cycloheptatriene.[17]
A Diels–Alder reaction with maleic anhydride forms the main ring system[18] and a subsequent reaction with 4-trifluormethylbenzhydrazide gives the cyclic imide of the drug.[17][19]
History
Originally researched by the National Institute of Allergy and Infectious Diseases, the drug was owned by Viropharma and discovered in collaboration with scientists at the United States Army Medical Research Institute of Infectious Diseases.[citation needed] It is owned and manufactured by SIGA Technologies.[citation needed] SIGA and Viropharma were issued a patent for tecovirimat in 2012.[20]
Clinical trials
As of 2009, the results of clinical trials support its use against smallpox and other related orthopoxviruses. It shows potential for a variety of uses including preventive healthcare, as a post-exposure therapeutic, as a therapeutic, and an adjunct to vaccination.[21][failed verification]
Tecovirimat can be taken by mouth and as of 2008, was permitted for phase II trials by the U.S. Food and Drug Administration (FDA). In phase I trials, tecovirimat was generally well tolerated with no serious adverse events.[22] Due to its importance for biodefense, the FDA designated tecovirimat for fast-track status, creating a path for expedited FDA review and eventual regulatory approval. On 13 July 2018, the FDA announced approval of tecovirimat for the treatment of smallpox.[23]
On 25 August 2022, the AIDS Clinical Trials Group (ACTG) began a randomized, placebo-controlled, double-blinded trial on the safety and efficacy of tecovirimat for mpox, known as STOMP (Study of Tecovirimat for Human mpox Virus), aiming to enroll at least 500 participants with acute mpox infection.[24][25]
Society and culture
Legal status
In November 2021, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization under exceptional circumstances for the medicinal product tecovirimat SIGA, intended for the treatment of orthopoxvirus disease (smallpox, mpox, cowpox, and vaccinia complications) in adults and in children who weigh at least 13 kilograms (29 lb)[26] The applicant for this medicinal product is SIGA Technologies Netherlands B.V.[26] Tecovirimat was approved for medical use in the European Union in January 2022.[5][27][28]
In December 2021, Health Canada approved oral tecovirimat for the treatment of smallpox in people weighing at least 13 kilograms (29 lb).[1][29]
As of August 2022, Tpoxx is available in the US only through the Strategic National Stockpile as a Centers for Disease Control and Prevention investigational new drug.[30][31] Intravenous Tpoxx has no lower weight cap and can be used in infants under the investigational new drug protocol.[32]
References
- ↑ 1.0 1.1 "Notice: Multiple Additions to the Prescription Drug List (PDL) [2022-01-24"]. 24 January 2022. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/multiple-additions-2022-01-24.html.
- ↑ "New Medicines Approved in 2018". 15 January 2020. https://www.canada.ca/en/patented-medicine-prices-review/services/npduis/analytical-studies/meds-entry-watch-2018/new-medicines-approved-2018.html.
- ↑ "Summary Basis of Decision (SBD) for Tpoxx". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00578&lang=en.
- ↑ 4.0 4.1 4.2 4.3 "Tpoxx- tecovirimat monohydrate capsule". 2 December 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fce826ab-4d6a-4139-a2ee-a304a913a253.
- ↑ 5.0 5.1 5.2 "Tecovirimat SIGA EPAR". 10 November 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/tecovirimat-siga. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 6.0 6.1 "Drug to Treat Smallpox Approved by F.D.A., a Move Against Bioterrorism". 13 July 2018. https://www.nytimes.com/2018/07/13/health/smallpox-drug-fda-bioterrorism.html.
- ↑ "Waking up to monkeypox". BMJ 377: o1321. May 2022. doi:10.1136/bmj.o1321. PMID 35613732.
- ↑ "Clinical features and management of human monkeypox: a retrospective observational study in the UK". The Lancet. Infectious Diseases 22 (8): 1153–1162. May 2022. doi:10.1016/S1473-3099(22)00228-6. PMID 35623380.
- ↑ "FDA approves the first drug with an indication for treatment of smallpox". U.S. Food and Drug Administration (FDA) (Press release). 13 July 2018. Archived from the original on 23 April 2019. Retrieved 1 August 2018.
- ↑ "U.S. Food and Drug Administration Approves Siga Technologies' Tpoxx (tecovirimat) for the Treatment of Smallpox". SIGA (Press release). Archived from the original on 21 September 2018. Retrieved 14 July 2018.
- ↑ "Oral Tecovirimat for the Treatment of Smallpox". The New England Journal of Medicine 379 (1): 44–53. July 2018. doi:10.1056/NEJMoa1705688. PMID 29972742.
- ↑ "Novel Treatment of a Vaccinia Virus Infection from an Occupational Needlestick - San Diego, California, 2019". MMWR. Morbidity and Mortality Weekly Report 68 (42): 943–946. October 2019. doi:10.15585/mmwr.mm6842a2. PMID 31647789. PMC 6812835. https://www.cdc.gov/mmwr/volumes/68/wr/pdfs/mm6842a2-H.pdf. Retrieved 2 August 2022.
- ↑ "Are we there yet? The smallpox research agenda using variola virus". PLOS Pathogens 10 (5): e1004108. May 2014. doi:10.1371/journal.ppat.1004108. PMID 24789223.
- ↑ "FDA approves the first smallpox treatment". 13 July 2018. https://www.sciencenews.org/article/first-smallpox-treatment-one-step-closer-fda-approval.
- ↑ (PDF) New Drug Therapy Approvals 2018 (Report). January 2019. https://www.fda.gov/media/120357/download. Retrieved 16 September 2020.
- ↑ "An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge". Journal of Virology 79 (20): 13139–13149. October 2005. doi:10.1128/JVI.79.20.13139-13149.2005. PMID 16189015.
- ↑ 17.0 17.1 ; Jordan, Robert & Rippin, Susan R."Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases" AU patent 2004249250, published 2004-12-29, assigned to SIGA Pharmaceuticals Inc
- ↑ "Re-examination of the Cycloaddition of Cycloheptatriene with Maleic Anhydride". Bulletin of the Chemical Society of Japan 44 (11): 2993–3000. 1971. doi:10.1246/bcsj.44.2993.
- ↑ "Review of the Patent Literature: Synthesis and Final Forms of Antiviral Drugs Tecovirimat and Baloxavir Marboxil". Organic Process Research & Development 23 (7): 1298–1307. 2019. doi:10.1021/acs.oprd.9b00144.
- ↑ U.S. Patent 8,124,643
- ↑ "SIGA Technologies". http://www.siga.com/index.php?ID=9.
- ↑ "Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor". Antimicrobial Agents and Chemotherapy 52 (5): 1721–1727. May 2008. doi:10.1128/AAC.01303-07. PMID 18316519.
- ↑ Office of the Commissioner (24 March 2020). "Press Announcements - FDA approves the first drug with an indication for treatment of smallpox". https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm613496.htm.
- ↑ National Institute of Allergy and Infectious Diseases (NIAID) (12 September 2022). "A Randomized, Placebo-Controlled, Double-Blinded Trial of the Safety and Efficacy of Tecovirimat for the Treatment of Human Monkeypox Virus Disease". https://clinicaltrials.gov/ct2/show/NCT05534984.
- ↑ "Trial Launching to Study Tecovirimat as Monkeypox Treatment" (in en). 16 September 2022. https://www.contagionlive.com/view/trial-launching-to-study-tecovirimat-as-monkeypox-treatment.
- ↑ 26.0 26.1 "Tecovirimat SIGA: Pending EC decision". 11 November 2021. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/tecovirimat-siga. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Summary of Product Characteristics". https://www.ema.europa.eu/en/documents/product-information/tecovirimat-siga-epar-product-information_en.pdf.
- ↑ "Tecovirimat SIGA Product information". https://ec.europa.eu/health/documents/community-register/html/h1600.htm.
- ↑ "SIGA Announces Health Canada Regulatory Approval of Oral Tpoxx" (Press release). Siga Technologies. 1 December 2021. Archived from the original on 24 May 2022. Retrieved 24 May 2022.
- ↑ "Information for Healthcare Providers on Obtaining and Using TPOXX (Tecovirimat) for Treatment of Monkeypox". 22 July 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/obtaining-tecovirimat.html.
- ↑ "Steps for Clinicians to Order Medication to Treat Monkeypox". Coca Now. 19 July 2022. https://emergency.cdc.gov/newsletters/coca/071922.htm. Retrieved 24 July 2022.
- ↑ "Monkeypox Outbreak: Updates on the Epidemiology, Testing, Treatment, and Vaccination". https://emergency.cdc.gov/coca/ppt/2022/072622_slides.pdf.
External links
- "Tecovirimat". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tecovirimat.
- "Tecovirimat monohydrate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tecovirimat%20monohydrate.
 |