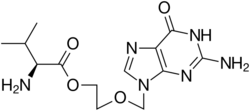
Chemistry:Valaciclovir
 | |
| Clinical data | |
|---|---|
| Trade names | Valtrex, Zelitrex, others |
| Other names | valacyclovir, valacyclovir hydrochloride (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695010 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 55% |
| Protein binding | 13–18% |
| Metabolism | Liver (to aciclovir) |
| Elimination half-life | <30 minutes (valaciclovir); 2.5–3.6 hours (aciclovir) |
| Excretion | Kidney 40–50% (aciclovir), faecal 47% (aciclovir) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| NIAID ChemDB | |
| Chemical and physical data | |
| Formula | C13H20N6O4 |
| Molar mass | 324.341 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Valaciclovir, also spelled valacyclovir, is an antiviral medication used to treat outbreaks of herpes simplex or herpes zoster (shingles).[2] It is also used to prevent cytomegalovirus following a kidney transplant in high risk cases.[2] It is taken by mouth.[2]
Common side effects include headache and vomiting.[2] Severe side effects may include kidney problems.[2] Use in pregnancy appears to be safe.[2] It is a prodrug, which works after being converted to aciclovir in a person's body.[2]
Valaciclovir was patented in 1987 and came into medical use in 1995.[3][4] It is on the World Health Organization's List of Essential Medicines.[5] It is available as a generic medication.[6] In 2020, it was the 119th most commonly prescribed medication in the United States, with more than 5 million prescriptions.[7][8]
Medical uses

Valaciclovir is used for the treatment of HSV and VZV infections, including:[9]
- Oral and genital herpes simplex (treatment and prevention)
- Reduction of HSV transmission from people with recurrent infection to uninfected individuals
- Herpes zoster (shingles): the typical dosage for treatment of herpes is 1,000 mg orally three times a day for seven consecutive days.[10]
- Prevention of cytomegalovirus following organ transplantation
- Prevention of herpesviruses in immunocompromised people (such as those undergoing cancer chemotherapy)[11]
- Chickenpox in children (ages 2–18)[1]
It has shown promise as a treatment for infectious mononucleosis[12][13][14] and is preventively administered in suspected cases of herpes B virus exposure.[15]
Bell's palsy does not seem to benefit from using valaciclovir as its only treatment.[16][17]
Adverse effects
Common adverse drug reactions (≥1% of people) associated with valaciclovir are the same as for aciclovir, its active metabolite. They include: nausea, vomiting, diarrhea and headache. Infrequent adverse effects (0.1–1% of patients) include: agitation, vertigo, confusion, dizziness, edema, arthralgia, sore throat, constipation, abdominal pain, rash, weakness and/or renal impairment. Rare adverse effects (<0.1% of patients) include: coma, seizures, neutropenia, leukopenia, tremor, ataxia, encephalopathy, psychotic symptoms, crystalluria, anorexia, fatigue, hepatitis, Stevens–Johnson syndrome, toxic epidermal necrolysis and/or anaphylaxis.[9]
Pharmacology
Valaciclovir is a prodrug, an esterified version of aciclovir that has greater oral bioavailability (about 55%) than aciclovir.[1] It is converted by esterases to the active drug, aciclovir, and the amino acid valine via hepatic first-pass metabolism. Aciclovir is selectively converted into a monophosphate form by viral thymidine kinase, which is more effective (3000 times) in phosphorylation of aciclovir than cellular thymidine kinase. Subsequently, the monophosphate form is further phosphorylated into a disphosphate by cellular guanylate kinase and then into the active triphosphate form, aciclo-GTP, by cellular kinases.[1]
Mechanism of action
Aciclo-GTP, the active triphosphate metabolite of aciclovir, is a very potent inhibitor of viral DNA replication. Aciclo-GTP competitively inhibits and inactivates the viral DNA polymerase.[1] Its monophosphate form also incorporates into the viral DNA, resulting in chain termination. It has also been shown that the viral enzymes cannot remove aciclo-GMP from the chain, which results in inhibition of further activity of DNA polymerase. Aciclo-GTP is fairly rapidly metabolized within the cell, possibly by cellular phosphatases.[18]
Aciclovir is active against most species in the herpesvirus family. In descending order of activity:[19]
- Herpes simplex virus type I (HSV-1)
- Herpes simplex virus type II (HSV-2)
- Varicella zoster virus (VZV)
- Epstein–Barr virus (EBV)
- Cytomegalovirus (CMV)
The drug is predominantly active against HSV and, to a lesser extent, VZV. It is only of limited efficacy against EBV and CMV. However, valaciclovir has been shown to lower or eliminate the presence of the Epstein–Barr virus in subjects afflicted with acute mononucleosis, leading to a significant decrease in the severity of symptoms.[12][13][14] Valaciclovir and acyclovir act by inhibiting viral DNA replication, but as of 2016 there was little evidence that they are effective against Epstein–Barr virus.[20] Acyclovir therapy does prevent viral latency, but has not proven effective at eradicating latent viruses in nerve ganglia.[19]
As of 2005, resistance to valaciclovir has not been significant. Mechanisms of resistance in HSV include deficient viral thymidine kinase and mutations to viral thymidine kinase and/or DNA polymerase that alter substrate sensitivity.[21]
It also is used for herpes B virus postexposure prophylaxis.[15][22]
Chemistry
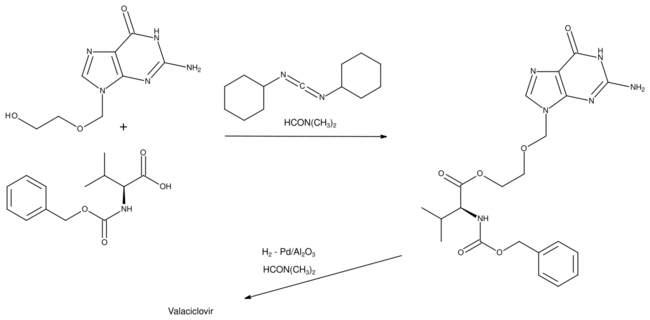
Details of the synthesis of valaciclovir were first published by scientists from the Wellcome Foundation.
Aciclovir was esterified with a carboxybenzyl protected valine, using dicyclohexylcarbodiimide as the dehydrating agent. In the final step, the protecting group was removed by hydrogenation using a palladium on alumina catalyst.[23][24]
History
Valaciclovir was patented in 1987 and came into medical use in 1995.[3][4] It is available as a generic medication.[6] In 2019, it was the 110th most commonly prescribed medication in the United States, with more than 5 million prescriptions.[7][8]
Society and culture
Brand names
It is marketed by GlaxoSmithKline under the brand names Valtrex[1] and Zelitrex. Valaciclovir has been available as a generic drug in the U.S. since November 2009.[25]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Valtrex- valacyclovir hydrochloride tablet, film coated". 14 June 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f8e0d8f8-cb73-4206-a484-88f5c4fbd719.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Valacyclovir Hydrochloride Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/valacyclovir-hydrochloride.html.
- ↑ 3.0 3.1 Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. 2012. p. 1502. ISBN 978-1437727029. https://books.google.com/books?id=nQ7-o8JAH7kC&pg=PA1502.
- ↑ 4.0 4.1 Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 504. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA504.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ 6.0 6.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 625–626. ISBN 9780857113382.
- ↑ 7.0 7.1 "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ 8.0 8.1 "Valacyclovir - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Valacyclovir.
- ↑ 9.0 9.1 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3[page needed]
- ↑ "Antiviral therapies of shingles in dermatology". Herpes zoroster: recent aspects of diagnosis and control. Monographs in virology. 26. Basel (Switzerland): Karger Publishers. 2006. p. 124. ISBN 978-3-8055-7982-7. https://books.google.com/books?id=3Eh51Np2w7IC&q=valacyclovir&pg=PA124. Retrieved 1 January 2012.
- ↑ "A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea". Support Care Cancer 18 (8): 993–1006. August 2010. doi:10.1007/s00520-010-0900-3. PMID 20544224.
- ↑ 12.0 12.1 "A controlled trial of valacyclovir in infectious mononucleosis.". 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC. December 2005. pp. 16–19. Abstract V1392.
- ↑ 13.0 13.1 "The Effect of Valacyclovir and Prednisolone in Reducing Symptoms of EBV Illness In Children: A Double-Blind, Placebo-Controlled Study". International Pediatrics 18 (3): 164–169. March 2003. https://www.researchgate.net/publication/237508616.
- ↑ 14.0 14.1 "A virologic pilot study of valacyclovir in infectious mononucleosis". Journal of Clinical Virology 39 (1): 16–21. May 2007. doi:10.1016/j.jcv.2007.02.002. PMID 17369082.
- ↑ 15.0 15.1 "Herpes B Virus: Information For Healthcare Providers". 31 January 2019. https://www.cdc.gov/herpesbvirus/healthcare-providers.html.
- ↑ "Clinical practice guideline: Bell's palsy". Otolaryngology–Head and Neck Surgery 149 (3_suppl): S1–S27. November 2013. doi:10.1177/0194599813505967. PMID 24189771.
- ↑ "Antiviral treatment for Bell's palsy (idiopathic facial paralysis)". Cochrane Database Syst Rev 2019 (9): CD001869. September 2019. doi:10.1002/14651858.CD001869.pub9. PMID 31486071.
- ↑ "Valaciclovir (VCV) - USCN LIFE SCIENCE INC". http://www.uscnk.us/protein-antibody-elisa/Valaciclovir-%28VCV%29-V511.htm.
- ↑ 19.0 19.1 O'Brien JJ, Campoli-Richards DM (March 1989). "Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy". Drugs 37 (3): 233–309. doi:10.2165/00003495-198937030-00002. PMID 2653790.
- ↑ "Antiviral agents for infectious mononucleosis (glandular fever)". The Cochrane Database of Systematic Reviews 2016 (12): CD011487. 2016. doi:10.1002/14651858.CD011487.pub2. PMID 27933614.
- ↑ Martindale: the complete drug reference (34th ed.). London: Pharmaceutical Press. 2005. ISBN 0-85369-550-4. OCLC 56903116.[page needed]
- ↑ "Recommendations for prevention of and therapy for exposure to B virus (cercopithecine herpesvirus 1)". Clin Infect Dis 35 (10): 1191–203. November 2002. doi:10.1086/344754. PMID 12410479.
- ↑ & Beauchamp, Lilia Marie"Therapeutic nucleosides" EP patent 308065, published 1989-03-22, assigned to Wellcome Foundation
- ↑ "34: Antiviral Drugs". Synthesis of Best-Seller Drugs. 2016. p. 709. doi:10.1016/B978-0-12-411492-0.00034-1. ISBN 9780124114920.
- ↑ "Ranbaxy Launches Generic Valtrex in U.S.". The Wall Street Journal. 27 November 2009. https://www.wsj.com/articles/SB125931422280866181.
External links
- "Valaciclovir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/valaciclovir.
- "Valacyclovir hydrochloride". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/valacyclovir%20hydrochloride.
 |