Chemistry:Tetraethynylmethane
From HandWiki
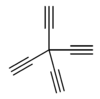
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,3-diethynylpenta-1,4-diyne | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C9H4 | |
| Molar mass | 112.131 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Tetraethynylmethane is an organic compound with formula C9H4, consisting of four ethynyl groups bonded to a central carbon atom. It is an alkyne, and one of the most compact possible hydrocarbons. It has been synthesised for potential applications in the synthesis of polymeric forms of carbon such as synthetic diamond and fullerenes.[1][2][3][4][5][6]
See also
- Neopentane
- Tetraethylmethane
- Tetravinylmethane
- Tetra-tert-butylmethane
- Tetracyclopropylmethane
- Tetraphenylmethane
- Tetrakis(trimethylsilyl)methane
- Methanetetracarboxylate
- Tetramethoxymethane
- Tetrafluoromethane
- Tetrachloromethane
- Tetrabromomethane
- Tetraiodomethane
- Tetraazidomethane
- Tetracyanomethane
- Tetranitromethane
References
- ↑ "Tetraethynylmethane.". Journal of the American Chemical Society 115 (9): 3846–3847. May 1993. doi:10.1021/ja00062a089.
- ↑ "Preparation and some subsequent transformations of tetraethynylmethane.". Journal of the American Chemical Society 116 (20): 9019–9026. October 1994. doi:10.1021/ja00099a020.
- ↑ "Preliminary studies towards crosslinking polymerizations with tetraethynylmethane and its derivatives: evidence for unusually facile alkyne hydration.". Chemical Communications (7): 865–866. January 1996. doi:10.1039/CC9960000865.
- ↑ Amemiya R, et al. One-Step Synthesis of Triethynylvinylmethanes and Tetraethynylmethanes by GaCl 3 -Promoted Diethynylation of 1,4-Enynes and 1,4-Diynes. Journal of the American Chemical Society 2005; 127(23):8252-3. doi:10.1021/ja051416l
- ↑ Zhao Z, et al. A 3D Organically Synthesized Porous Carbon Material for Lithium-Ion Batteries. Angewandte Chemie 2018; 57(37): 11952-11956. doi:10.1002/anie.201805924
- ↑ Kilde MD, Murray AH, Andersen CL, et al. Synthesis of radiaannulene oligomers to model the elusive carbon allotrope 6,6,12-graphyne. Nat Commun 2019;10:3714. doi:10.1038/s41467-019-11700-0
 |

