Chemistry:Tetramethoxymethane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetramethoxymethane | |
| Other names
Tetramethyl orthocarbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3272 |
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 g·mol−1 |
| Appearance | colourless liquid[1] |
| Density | 1.023 g/cm3 (25 °C) |
| Melting point | −5.5 °C[1] |
| Boiling point | 114 °C[1] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H315, H319, H335 | |
| P210, P261, P305+351+338 | |
| Related compounds | |
Other cations
|
Tetramethoxysilane |
Related compounds
|
Tetraethoxymethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetramethoxymethane is a chemical compound which is formally formed by complete methylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarboxylic acid violates the Erlenmeyer rule and is unstable in free state).
Preparation
The obvious synthetic route from tetrachloromethane does not yield the desired product.[2] The original preparation of the tetramethoxymethane was therefore based on chloropicrin:[1]
Because of the unpleasant properties of the chloropicrin, other tetrasubstituted reactive methane derivatives were investigated as starting material for tetramethoxymethane. For example, trichloromethanesulfenyl chloride (also used as a chemical warfare agent and easily accessible from carbon bisulfide and chlorine) was used:[3][4]
A less problematic synthesis is based on trichloroacetonitrile,[5][6] with yields of about 70% be achieved:
Further preparative methods are described in the literature.[7]
Properties
Tetramethoxymethane is water-clear, aromatic-smelling, low-viscosity liquid which is stable against peroxide formation.[8]
Use
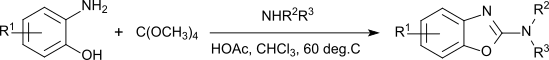
In addition to the use as a solvent, tetramethoxymethane is used as a fuel in polymer fuel cells,[9] as an alkylating agent at elevated temperatures (180-200 °C)[10] as a transesterification reagent (but showing less reactivity than trimethoxymethane[2]) and as a reagent for the synthesis of 2-aminobenzoxazoles, which are used as molecular building blocks in pharmaceutical active ingredients used in neuroleptics, sedatives, antiemetics, muscle relaxants, fungicides and others.[11]
Depending on the substituents, the one pot reaction proceeds in "modest to excellent" yields.
References
- ↑ 1.0 1.1 1.2 1.3 H. v. Hartel, Über Existenz und Darstellung des Orthokohlensäure-tetramethylesters, Ber.dtsch.chem.Ges., 60(8), 1841 (1927), doi:10.1002/cber.19270600821.
- ↑ 2.0 2.1 R. H. De Wolfe, Carboxylic ortho acid derivatives: preparation and synthetic applications, Organic Chemistry, Vol. 14, Academic Press, Inc. New York – London, 1970, ISBN 978-0-12-214550-6.
- ↑ H. Tieckelmann, H. W. Post, The preparation of methyl, ethyl, propyl, and butyl orthocarbonates, J. Org. Chem., 13 (2), 265-267 (1948), doi:10.1021/jo01160a014.
- ↑ US-Patent US 4,059,656, Processes for neutralizing 2,3-dibromopropanol phosphoric acid esters contained in tris(2,3-dibromo-1-propyl) phosphate, Erfinder: M. Demarcq, Anmelder: Produits Chimiques Ugine Kuhlmann, erteilt am 22. November 1974.
- ↑ US-Patent US 3,876,708, Orthocarbonic acid esters, Erfinder: R. Speh, W. Kantlehner, Anmelder: Akzo B.V., erteilt am 8. April 1975.
- ↑ US-Patent US 6,825,385 B2, Process for the preparation of orthocarbonates, Erfinder: G. Fries, J. Kirchhoff, Anmelder: Degussa AG, erteilt am 30. November 2004.
- ↑ W. Kantlehner et al., Die präparative Chemie der O- und N-funktionellen Orthokohlensäure-Derivate, Synthesis; 1977(2): 73-90, doi:10.1055/s-1977-24283.
- ↑ K. R. Kopecky; J. Molina (1987). "Bis(dimethoxymethyl) peroxide and bis(1,1-dimethoxyethyl) peroxide". Canadian Journal of Chemistry 65 (10): 2350. doi:10.1139/v87-392.
- ↑ US-Patent US 6,864,001, Tetramethyl orthocarbonate fuel cells and systems and methods related thereto, Erfinder: J. Zhang, K. Colbow, Anmelder: Ballard Power Systems Inc., erteilt am 8. März 2005.
- ↑ M. Selva et al., Esters and Orthoesters as Alkylating Agents at High Temperature. Applications to Continuous-flow Processes, J. Chem. Soc., Perkin Trans. 2, 519 (1992), doi:10.1039/P29920000519.
- ↑ C. L. Cioffi et al., Synthesis of 2-Aminobenzoxazoles Using Tetramethyl Orthocarbonate or 1,1-Dichloro-diphenoxymethane, J. Org. Chem., 75 (2), 7942-7945 (2010), doi:10.1021/jo1017052.
 |