Chemistry:Tetraphenylphosphonium chloride
| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetraphenylphosphanium chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| [P(C 6H 5) 4]Cl | |
| Molar mass | 374.85 g·mol−1 |
| Appearance | colourless solid |
| Density | 1.27 g dm−3 |
| Melting point | 272 to 274 °C (522 to 525 °F; 545 to 547 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetraphenylphosphonium chloride is the chemical compound with the formula [(C
6H
5)
4P]Cl, abbreviated Ph
4PCl or PPh
4Cl or [PPh
4]Cl, where Ph stands for phenyl. Tetraphenylphosphonium and especially tetraphenylarsonium salts were formerly of interest in gravimetric analysis of perchlorate and related oxyanions. This colourless salt is used to generate lipophilic salts from inorganic and organometallic anions. Thus, [Ph
4P]+
is useful as a phase-transfer catalyst, again because it allows inorganic anions to dissolve in organic solvents.
Structure and basic properties
The structure of this salt is [PPh
4]+
Cl−
. It consists of tetraphenylphosphonium cations [PPh
4]+
and chloride anions Cl−
. The [PPh
4]+
cation is tetrahedral.
PPh4Cl crystallises as the anhydrous salt,[2] which is the normal item of commerce, as well as a monohydrate[3] and a dihydrate.[4]
In X-ray crystallography, PPh+
4 salts are of interest as they often crystallise easily. The rigidity of the phenyl groups facilitates packing and elevates the melting point relative to alkyl-based quaternary ammonium salts. Also, since these salts are soluble in organic media, a wide range of solvents can be employed for their crystallisation.
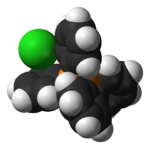 |
200px | 
|
| constituent ions in the solid | space-filling model of part of the crystal structure |
ball-and-stick model of part of the crystal structure |
Preparation
[PPh
4]Cl and many analogous compounds can be prepared by the reaction of chlorobenzene with triphenylphosphine catalysed by nickel salts:[5]
- PhCl + PPh
3 → [Ph
4P]Cl
The compound was originally prepared as the corresponding bromide salt (CAS No. 2751-90-8), which in turn was synthesized by passing dry oxygen through the reaction of phenylmagnesium bromide and triphenylphosphine.[6] The synthesis probably proceeds via the reaction of the Grignard reagent with triphenylphosphine oxide.
- PhMgBr + Ph
3PO → [Ph
4P]OMgBr - [Ph
4P]OMgBr + HBr → [Ph
4P]Br + "Mg(OH)Br"
Use in synthesis
Tetraphenylphosphonium salts of inorganic or organometallic anions are often sought because they are easily crystallized. They also tend to be soluble in polar organic solvents such as acetonitrile and dimethylformamide. Examples include the tetraphenylphosphonium perrhenate ([PPh
4]+
[ReO
4]−
)[7] and various thiomolybdates.[8] Complexes of maleonitriledithiolate are also isolated as their [PPh
4]+
salts.[9]
References
- ↑ "Tetraphenylphosphonium chloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/164911#section=Safety-and-Hazards.
- ↑ Richardson, J. F.; Ball, J. M.; Boorman, P. M. (1986). "Structure of Tetraphenylphosphonium Chloride". Acta Crystallographica C42 (9): 1271–1272. doi:10.1107/S0108270186092612.
- ↑ Schweizer, E. E.; Baldacchini, C. J.; Rheingold, A. L. (1989). "Tetraphenylphosphonium Chloride Monohydrate, Tetraphenylphosphonium Bromide and Tetraphenylphosphonium Iodide". Acta Crystallographica C45 (8): 1236–1239. doi:10.1107/S0108270189000363.
- ↑ Blake, A. J.; Garner, C. D.; Tunney, J. M. (2003). "Tetraphenylphosphonium Chloride Dihydrate". Acta Crystallographica E59: o9–o10. doi:10.1107/S1600536802021682.
- ↑ Marcoux, David; Charette, André B. (2008). "Nickel-Catalyzed Synthesis of Phosphonium Salts from Aryl Halides and Triphenylphosphine". Adv. Synth. Catal. 350 (18): 2967–2974. doi:10.1002/adsc.200800542.
- ↑ Dodonow, J.; Medox, H. (1928). "Zur Kenntnis der Grignardschen Reaktion: Über die Darstellung von Tetraphenyl-phosphoniumsalzen". Berichte der Deutschen Chemischen Gesellschaft 61 (5): 907–911. doi:10.1002/cber.19280610505.
- ↑ Dilworth, J. R.; Hussain, W.; Hutson, A. J.; Jones, C. J.; McQuillan, F. S. (1996). "Tetrahalo Oxorhenate Anions". Inorganic Syntheses. Inorganic Syntheses. XXXI. pp. 257–262. doi:10.1002/9780470132623.ch42. ISBN 9780470132623.
- ↑ Hadjikyriacou, A. I.; Coucouvanis, D. (1990). "Tetraphenylphosphonium Salts of [Mo2 (S) N (S2 )6-N ]2- Thioanions and Derivatives". Tetraphenylphosphonium Salts of [Mo2(S)n(S2)6-n]2- Thioanions and Derivatives. Inorganic Syntheses. pp. 39–47volume=XXVII. doi:10.1002/9780470132586.ch8. ISBN 9780470132586.
- ↑ Bray, J.; Locke, J.; McCleverty, J. A.; Coucouvanis, D. (1972). "Bis[cis -1,2-dicyanoethene-1,2-dithiolato(1- or 2-)] Complexes of Cobalt and Iron". Bis[cis-1,2-Dicyanoethene-1,2-dithiolato(1- or 2-)] Complexes of Cobalt and Iron. Inorganic Syntheses. XIII. pp. 187–195. doi:10.1002/9780470132449.ch39. ISBN 9780470132449.
 |
