Chemistry:Thymidine diphosphate glucose
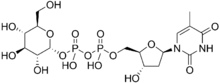
| |
| Names | |
|---|---|
| IUPAC name
Thymidine 5′-(α-D-glucopyranosyl trihydrogen diphosphate)
| |
| Systematic IUPAC name
O1-{[(2R,3S,5R)-3-Hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(3H)-yl)oxolan-2-yl]methyl} O3-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen diphosphate | |
| Other names
TDP-glucose; dTDP-glucose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C16H26N2O16P2 | |
| Molar mass | 564.330 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thymidine diphosphate glucose (often abbreviated dTDP-glucose or TDP-glucose) is a nucleotide-linked sugar consisting of deoxythymidine diphosphate linked to glucose. It is the starting compound for the syntheses of many deoxysugars.[1]
Biosynthesis
DTDP-glucose is produced by the enzyme glucose-1-phosphate thymidylyltransferase and is synthesized from dTTP and glucose-1-phosphate. Pyrophosphate is a byproduct of the reaction.
Uses within the cell
DTDP-glucose goes on to form a variety of compounds in nucleotide sugars metabolism. Many bacteria utilize dTDP-glucose to form exotic sugars that are incorporated into their lipopolysaccharides or into secondary metabolites such as antibiotics. During the syntheses of many of these exotic sugars, dTDP-glucose undergoes a combined oxidation/reduction reaction via the enzyme dTDP-glucose 4,6-dehydratase, producing dTDP-4-keto-6-deoxy-glucose.[1][2]
References
- ↑ 1.0 1.1 Xue M. He; Hung-wen Liu (2002). "Formation of unusual sugars: Mechanistic studies and biosynthetic applications". Annu Rev Biochem 71: 701–754. doi:10.1146/annurev.biochem.71.110601.135339. PMID 12045109.
- ↑ "Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly". Carbohydr. Res. 338 (23): 2503–19. 2003. doi:10.1016/j.carres.2003.07.009. PMID 14670712.
 |

