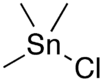
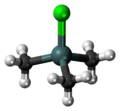
Chemistry:Trimethyltin chloride
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chlorotri(methyl)stannane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 3146 2786 | ||
| |||
| |||
| Properties | |||
| (CH 3) 3SnCl | |||
| Molar mass | 199.27 g·mol−1 | ||
| Appearance | White solid | ||
| Odor | Malodorous | ||
| Melting point | 38.5 °C (101.3 °F; 311.6 K)[1] | ||
| Boiling point | 148 °C (298 °F; 421 K) | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H300, H310, H330, H410 | |||
| P260, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P304+340, P310, P320, P322, P330, P361, P363, P391, P403+233, P405 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trimethyltin chloride is an organotin compound with the formula (CH
3)
3SnCl. It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis.
Synthesis
Trimethyltin chloride can be prepared by the redistribution reaction of tetramethyltin with tin tetrachloride.[3]
- SnCl
4 + 3 Sn(CH
3)
4 → 4 (CH
3)
3SnCl
This redistribution reaction is typically performed with no solvent because high temperatures are required and purification is simplified.
A second route to (CH
3)
3SnCl involves treating the corresponding hydroxide or oxide (in the following reaction, trimethyltin hydroxide (CH
3)
3SnOH) with a halogenating agent such as hydrogen chloride or thionyl chloride (SOCl
2):
- (CH
3)
3SnOH + HCl → (CH
3)
3SnCl + H
2O
Uses
Trimethyltin chloride is used as a source of the trimethylstannyl group ((CH
3)
3Sn–).[4] For example, it is a precursor to vinyltrimethylstannane ((CH
3)
3SnCH=CH
2)[5] and indenyltrimethylstanane (CH
3)
3SnC
9H
7 (see Transition metal indenyl complex):[6]
- CH
2=CHMgBr + (CH
3)
3SnCl → (CH
3)
3SnCH=CH
2 + MgBrCl - LiC
9H
7 + (CH
3)
3SnCl → (CH
3)
3SnC
9H
7 + LiCl
An example of an organolithium reagent reacting with (CH
3)
3SnCl to form a tin-carbon bond is:
- LiCH(Si(CH
3)
3)(Ge(CH
3)
3) + (CH
3)
3SnCl → (CH
3)
3SnCH(Si(CH
3)
3)(Ge(CH
3)
3) + LiCl
Organotin compounds derived from Me
3SnCl are useful in organic synthesis, especially in radical chain reactions. (CH
3)
3SnCl is a precursor to compounds used in PVC stabilization.
Reduction of trimethyltin chloride with sodium gives hexamethylditin:[7]
- 2 Na + 2 (CH
3)
3SnCl → (CH
3)
3Sn–Sn(CH
3)
3 + 2 NaCl
References
- ↑ Lide, D. R.; Milne, G. W. (1994). Handbook of Data on Organic Compounds. 4 (3rd ed.). CRC Press. p. 4973.
- ↑ "Trimethyltin chloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/14016#section=Safety-and-Hazards.
- ↑ Scott, W. J.; Crisp, G. T.; Stille, J. K. (1990). "Palladium-catalyzed Coupling of Vinyl Triflates with Organostannanes: 4-tert-Butylcyclohexen-1-yl)-2-propen-1-one". Organic Syntheses 68: 116. http://www.orgsyn.org/demo.aspx?prep=cv8p0097.; Collective Volume, 8, pp. 97
- ↑ Davies, A. G. (2008). "Tin Organometallics". Comprehensive Organometallic Chemistry. 3. Elsevier. pp. 809–883. doi:10.1016/B0-08-045047-4/00054-6. ISBN 9780080450476.
- ↑ William J. Scott; G. T. Crisp; J. K. Stille (1990). "Palladium-Catalyzed Coupling of Vinyl Triflates with Organostannanes: 4-tert-Butyl-1-vinylcyclohexene and 1-(4-tert-Butylcyclohexen-1-yl)-2-propen-1-one". Organic Syntheses 68: 116. doi:10.15227/orgsyn.068.0116.
- ↑ Robert J. Morris; Scott L. Shaw; Jesse M. Jefferis; James J. Storhoff; Dean M. Goedde (1998). "Monoindenyltrichloride Complexes of Titanium(IV), Zirconium(IV), and Hafnium(IV)". Inorganic Syntheses. Inorganic Syntheses. 32. pp. 215–221. doi:10.1002/9780470132630.ch36. ISBN 9780470132630.
- ↑ Eisch, John J. (1981). Organometallic Syntheses II. New York: Academic Press. pp. 167. ISBN 0-12-234950-4.
 |