Chemistry:Triphenyl phosphite ozonide
From HandWiki
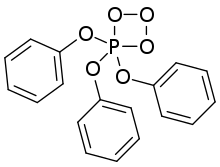
| |
| Names | |
|---|---|
| IUPAC name
4,4,4-triphenoxy-1,2,3,4λ5-trioxaphosphetane
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | TPPO |
PubChem CID
|
|
| |
| |
| Properties | |
| C18H15O6P | |
| Molar mass | 358.286 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triphenyl phosphite ozonide (TPPO) is a chemical compound with the formula PO3(C6H5O)3 that is used to generate singlet oxygen.[1][2]
When TPPO is mixed with amines, the ozonide breaks down into singlet oxygen and leaves behind triphenyl phosphite.[2] Pyridine is the only known amine that can effectively cause the breakdown of TPPO while not quenching any of the produced oxygen.[1][2]
Synthesis
Triphenyl phosphite ozonide is created by bubbling dry ozone through dichloromethane with triphenyl phosphite being added dropwise at -78 °C.[2] If triphenyl phosphite is added in excess in the synthesis, TPPO can be reduced to triphenyl phosphite oxide, PO(C6H5O)3, and oxygen gas.[3]
References
- ↑ 1.0 1.1 Pryor, William A.; Govindan, C. K. (November 1981). "Decomposition of triphenyl phosphite ozonide in the presence of spin traps" (in en). The Journal of Organic Chemistry 46 (23): 4679–4682. doi:10.1021/jo00336a010. ISSN 0022-3263. https://pubs.acs.org/doi/abs/10.1021/jo00336a010.
- ↑ 2.0 2.1 2.2 2.3 Bartlett, Paul D.; Mendenhall, G. David; Durham, Dana L. (October 1980). "Controlled generation of singlet oxygen at low temperatures from triphenyl phosphite ozonide" (in en). The Journal of Organic Chemistry 45 (22): 4269–4271. doi:10.1021/jo01310a001. ISSN 0022-3263. https://pubs.acs.org/doi/abs/10.1021/jo01310a001.
- ↑ Mendenhall, G. David; Priddy, Duane B. (1999-08-01). "A Reexamination of the Ozone−Triphenyl Phosphite System. The Origin of Triphenyl Phosphate at Low Temperatures" (in en). The Journal of Organic Chemistry 64 (16): 5783–5786. doi:10.1021/jo982339y. ISSN 0022-3263. https://pubs.acs.org/doi/10.1021/jo982339y.
 |

