Chemistry:Vanadyl isopropoxide
From HandWiki
| |
| Names | |
|---|---|
| Other names
Triisopropoxyvanadium(V) oxide; VTIP; Vanadium(V) trisisopropoxide oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H21O4V | |
| Molar mass | 244.205 g·mol−1 |
| Melting point | −14 to −11 °C (7 to 12 °F; 259 to 262 K) |
| Boiling point | 242 °C (468 °F; 515 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
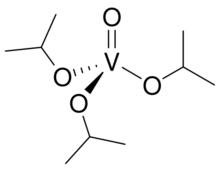
Vanadyl isopropoxide is the metal alkoxide with the formula VO(O-iPr)3 (iPr = CH(CH3)2). A yellow volatile liquid, it is a common alkoxide of vanadium. It is used as a reagent and as a precursor to vanadium oxides.[1] The compound is diamagnetic. It is prepared by alcoholysis of vanadyl trichloride:
- VOCl3 + 3 HOCH(CH3)2 → VO(OCH(CH3)2)3 + 3 HCl
The related cyclopentanoxide VO(O-CH(CH2)4)3 is a dimer, one pair of alkoxide ligands bind weakly trans to the vanadyl oxygens.[2]
References
- ↑ Krumeich, F.; Muhr, H.-J.; Niederberger, M.; Bieri, F.; Schnyder, B.; Nesper, R. (1999). "Morphology and Topochemical Reactions of Novel Vanadium Oxide Nanotubes". Journal of the American Chemical Society 121 (36): 8324–8331. doi:10.1021/ja991085a.
- ↑ Hillerns, Frank; Olbrich, Falk; Behrens, Ulrich; Rehder, Dieter (1992). "Tris(cyclopentanolato)oxovanadium(V): A Model for the Transition State of Enzymatic Phosphoester Cleavage". Angewandte Chemie International Edition in English 31 (4): 447–448. doi:10.1002/anie.199204471.
 |

