Chemistry:Yttrium oxalate
| |
| Names | |
|---|---|
| Other names
Yttrium(3+) oxalate, Diyttrium trioxalate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Y2(C2O4)3 | |
| Molar mass | 441.87 |
| Appearance | White crystals |
| insoluble | |
Solubility product (Ksp)
|
5.1 × 10−30.[1] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302+312Script error: No such module "Preview warning".Category:GHS errors, H302, H312[2] | |
| P264, P270, P280, P301+312, P302+352, P312, P322, P330, P363, P501 | |
| Related compounds | |
Related compounds
|
Calcium oxalate Sodium oxalate Magnesium oxalate Strontium oxalate Barium oxalate Iron(II) oxalate Iron(III) oxalate Lithium oxalate Praseodymium oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
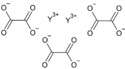
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3.[3] The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.[4]
Synthesis
Precipitation of soluble yttrium salts with oxalic acid:[5]
- [math]\displaystyle{ \mathsf{ 2YCl_3 + 3H_2C_2O_4 \ \xrightarrow{}\ Y_2(C_2O_4)_3\downarrow + 6HCl } }[/math]
Properties
Yttrium oxalate is highly insoluble in water and converts to the oxide when heated.[6] Yttrium oxalate forms crystalline hydrates (colorless crystals) with the formula Y2(C2O4)3•n H2O, where n = 4, 9, and 10.
Decomposes when heated:
- [math]\displaystyle{ \mathsf{ Y_2(C_2O_4)_3 \ \xrightarrow{700^oC}\ Y_2O_3 + 3CO_2 + 3CO } }[/math]
The solubility product of yttrium oxalate at 25 °C is 5.1 × 10−30.[1]
The trihydrate Y2(C2O4)3•3H2O is formed by heating more hydrated varieties at 110 °C.[7]
Y2(C2O4)3•2H2O, which is formed by heating the decahydrate at 210 °C) forms monoclinic crystals with unit cell dimensions a=9.3811 Å, b=11.638 Å, c=5.9726 Å, β=96.079°.[8]
Related
Several yttrium oxalate double salts are known containing additional cations. Also a mixed-anion compound with carbonate is known.
| formula | name | formula weight | crystal form | space group | unit cell Å | volume Å3 | properties | references |
|---|---|---|---|---|---|---|---|---|
| NH4Y(C2O4)2.H2O | Ammonium yttrium oxalate monohydrate | monoclinic | P2/n | a=9.18 b=6.09 c=7.89 β=90.2 Z=2 | [9] | |||
| [C6N2H16]0.5[Y(H2O)(C2O4)2]·2H2O | 377.1 | triclinic | P1 | a = 8.229, b = 9.739, c = 9.754, α = 60.74, β = 72.36, γ = 84.67°, Z = 2 | 648.5 | density 1.931 | [10] | |
| [C5N2H12] [Y(C2O4)2] | 365.1 | monoclinic | Cc | a = 11.552, b = 17.168, c = 8.719, β = 130.64°, Z = 2 | 1312.1 | density 1.848 | [10] | |
| C5NH6Y(C2O4)2•3H2O | Pyridinium yttrium oxalate trihydrate | [11] | ||||||
| [Y(H2O)]Na(C2O4)2.3H2O | yttrium sodium oxalate tetrahydrate | 360.005 | monoclinic | Pc | a=8.623 b=8.6310 c=14.896 β=102.848 Z=4 | 1080.9 | [12] | |
| YK(C2O4)2.4H2O | Yttrium potassium oxalate tetrahydrate | tetragonal | I41/a | Z = 4 a = 11.4612, c = 8.9040 | 1169.6 | [13][14] | ||
| Y(H2O)Cs(C2O4)2 | Caesium yttrium oxalate monohydrate | monoclinic | P2/n | a = 8.979, b = 6.2299, c = 8.103, β = 90.05° | 453.3 | [15] | ||
| RbLn(C2O4)2•3H2O | Rubidium yttrium oxalate trihydrate | [16] | ||||||
| [Y(H2O)]2(C2O4)(CO3)2 | yttrium oxalate carbonate | 421.876 | orthorhombic | C2221 | a = 7.8177, b = 14.943, c = 9.4845, Z = 4 | 1108.0 | density 2.526 | [17] |
References
- ↑ Jump up to: 1.0 1.1 Chung, Dong-Yong; Kim, Eung-Ho; Lee, Eil-Hee; Yoo, Jae-Hyung (1998). "Solubility of Rare Earth Oxalate in Oxalic and Nitric Acid Media" (in ko). Journal of Industrial and Engineering Chemistry 4 (4): 277–284. https://www.cheric.org/research/tech/periodicals/view.php?seq=12719.
- ↑ "Yttrium Oxalate | Diyttrium trioxalate | C6H18O21Y2". Ereztech. https://ereztech.com/product/yttrium-oxalate-13266-82-5/.
- ↑ "Yttrium oxalate" (in en). National Institute of Standards and Technology. https://webbook.nist.gov/cgi/cbook.cgi?ID=B6000081&Mask=80.
- ↑ Feibush, A. M.; Rowley, Keith; Gordon, Louis (1958-10-01). "Solubility of Yttrium Oxalate". Analytical Chemistry 30 (10): 1610–1612. doi:10.1021/ac60142a008. https://pubs.acs.org/doi/abs/10.1021/ac60142a008. Retrieved 25 June 2021.
- ↑ Yoon, Jung Hoon (1990) (in en). Precipitation of Yttrium and Rare Earth Powders from Aqueous Solutions and Emulsions. University of California, Berkeley. p. 54. https://books.google.com/books?id=wZlLAQAAMAAJ&q=yttrium+oxalate. Retrieved 25 June 2021.
- ↑ "Yttrium Oxalate" (in en). American Elements. https://www.americanelements.com/yttrium-oxalate-13266-82-5.
- ↑ Moosath, S. S.; Abraham, John; Swaminathan, T. V. (August 1963). "Thermal Decomposition of Rare Earth Metal Oxalates. III. Oxalates of Holmium, Erbium, Thulium, Ytterbium, Lutetium and Yttrium". Zeitschrift für anorganische und allgemeine Chemie 324 (1–2): 99–102. doi:10.1002/zaac.19633240112.
- ↑ Louër, D.; Deneuve, F.; Ecochard, F. (June 1990). "Indexing of the Powder Diffraction Pattern of Yttrium Oxalate Dihydrate". Powder Diffraction 5 (2): 104–105. doi:10.1017/S0885715600015438. Bibcode: 1990PDiff...5..104L.
- ↑ McDonald, T. R. R.; Spink, J. M. (1967-12-10). "The crystal structure of a double oxalate of yttrium and ammonium, NH4Y(C2O4)2.H2O". Acta Crystallographica 23 (6): 944–949. doi:10.1107/S0365110X67004104. http://scripts.iucr.org/cgi-bin/paper?S0365110X67004104.
- ↑ Jump up to: 10.0 10.1 Vaidhyanathan, R.; Natarajan, Srinivasan; Rao, C. N. R. (January 2001). "Three-Dimensional Yttrium Oxalates Possessing Large Channels" (in en). Chemistry of Materials 13 (1): 185–191. doi:10.1021/cm000419o. ISSN 0897-4756. https://pubs.acs.org/doi/10.1021/cm000419o.
- ↑ Genčova, O.; Šiftar, J. (1994). "Synthesis and Thermal Analysis of Double Oxalates of Rare Earths (III) with the Pyridinium Cation". Bulletin of the Chemists and Technologists of Macedonia 13: 25–28. https://repository.ukim.mk/handle/20.500.12188/11354.
- ↑ Bataille, T.; Louër, D. (1999-11-15). "Yttrium sodium oxalate tetrahydrate, [Y(H 2 O)]Na(C 2 O 4 ) 2 . 3H 2 O". Acta Crystallographica Section C: Crystal Structure Communications 55 (11): 1760–1762. doi:10.1107/S0108270199009683.
- ↑ Bataille, T.; Auffrédic, J.-P.; Louër, D. (January 2000). "A Powder Diffraction Study of the Crystal Structure and the Dehydration Process of Yttrium Potassium Oxalate Tetrahydrate". Materials Science Forum 321-324: 976–981. doi:10.4028/www.scientific.net/MSF.321-324.976.
- ↑ Zhang, Xingjing; Xing, Yongheng; Wang, Chunguang; Han, Jing; Li, Jing; Ge, Maofa; Zeng, Xiaoqing; Niu, Shuyun (March 2009). "Lanthanide–alkali metals–oxalates coordination polymers: Synthesis and structures of [Nd(C2O4)1.5(H2O)3]·2H2O, Nd(C2O4)(CH3COO)(H2O), KLn(C2O4)2(H2O)4 (Ln=Y, Tb)". Inorganica Chimica Acta 362 (4): 1058–1064. doi:10.1016/j.ica.2008.05.029.
- ↑ Bataille, Thierry; Auffrédic, Jean-Paul; Louër, Daniel (2000). "Crystal structure and thermal behaviour of the new layered oxalate Y(H2O)Cs(C2O4)2 studied by powder X-ray diffraction". Journal of Materials Chemistry 10 (7): 1707–1711. doi:10.1039/b001201g. http://xlink.rsc.org/?DOI=b001201g.
- ↑ Genčova, O.; šiftar, J. (April 1997). "Synthesis and dehydration of double oxalates of rare earths(III) with some monovalent metals: II. Investigations of RbLn(C2O4)2·nH2O". Journal of Thermal Analysis 48 (4): 877–884. doi:10.1007/bf01997192.
- ↑ Bataille, Thierry; Louër, Daniel (2000-12-01). "Powder and single-crystal X-ray diffraction study of the structure of [Y(H 2 O)] 2 (C 2 O 4 )(CO 3 ) 2". Acta Crystallographica Section B: Structural Science 56 (6): 998–1002. doi:10.1107/S0108768100010004. PMID 11099966.
 |

