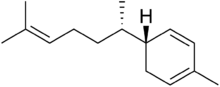
Chemistry:Zingiberene
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-5-(6-methylhept-5-en-2-yl)cyclohexa-1,3-diene | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 2554989 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | zingiberene |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
| Density | 871.3 mg cm−3 (at 20 °C) |
| Boiling point | 134 to 135 °C (273 to 275 °F; 407 to 408 K) at 2.0 kPa |
| log P | 6.375 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zingiberene is a monocyclic sesquiterpene that is the predominant constituent of the oil of ginger (Zingiber officinale),[1] from which it gets its name. It can contribute up to 30% of the essential oils in ginger rhizomes. This is the compound that gives ginger its distinct flavoring.
Biosynthesis
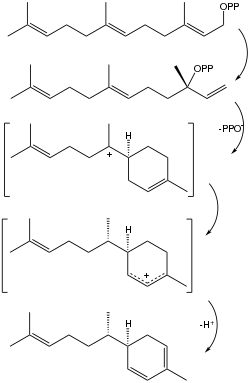
Zingiberene is formed in the isoprenoid pathway from farnesyl pyrophosphate (FPP). FPP undergoes a rearrangement to give nerolidyl diphosphate. After the removal of pyrophosphate, the ring closes leaving a carbocation on the tertiary carbon attached to the ring. A 1,3-hydride shift then takes place to give a more stable allylic carbocation. The final step in the formation of zingiberene is the removal of the cyclic allylic proton and consequent formation of a double bond. Zingiberene synthase is the enzyme responsible for catalyzing the reaction forming zingiberene as well as other mono- and sesquiterpenes.[2]
See also
- Gingerol
- sequiphellandrene
References
- ↑ 1.0 1.1 Herout, Vlastimil; Benesova, Vera; Pliva, Josef (1953). "Terpenes. XLI. Sesquiterpenes of ginger oil". Collection of Czechoslovak Chemical Communications 18: 297–300. doi:10.1135/cccc19530248.
- ↑ K. Rani (1999). "Cyclisation of farnesyl pyrophosphate into sesquiterpenoids in ginger rhizomes ("Zingiber officinale")". Fitoterapia 70 (6): 568–574. doi:10.1016/S0367-326X(99)00090-8.
 |