Earth:Atmospheric escape
Atmospheric escape is the loss of planetary atmospheric gases to outer space. A number of different mechanisms can be responsible for atmospheric escape; these processes can be divided into thermal escape, non-thermal (or suprathermal) escape, and impact erosion. The relative importance of each loss process depends on the planet's escape velocity, its atmosphere composition, and its distance from its star. Escape occurs when molecular kinetic energy overcomes gravitational energy; in other words, a molecule can escape when it is moving faster than the escape velocity of its planet. Categorizing the rate of atmospheric escape in exoplanets is necessary to determining whether an atmosphere persists, and so the exoplanet's habitability and likelihood of life.
Thermal escape mechanisms
Thermal escape occurs if the molecular velocity due to thermal energy is sufficiently high. Thermal escape happens at all scales, from the molecular level (Jeans escape) to bulk atmospheric outflow (hydrodynamic escape).
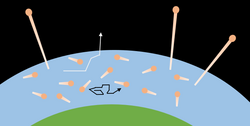
Jeans escape
One classical thermal escape mechanism is Jeans escape,[1] named after British astronomer Sir James Jeans, who first described this process of atmospheric loss.[2] In a quantity of gas, the average velocity of any one molecule is measured by the gas's temperature, but the velocities of individual molecules change as they collide with one another, gaining and losing kinetic energy. The variation in kinetic energy among the molecules is described by the Maxwell distribution. The kinetic energy (), mass (), and velocity () of a molecule are related by . Individual molecules in the high tail of the distribution (where a few particles have much higher speeds than the average) may reach escape velocity and leave the atmosphere, provided they can escape before undergoing another collision; this happens predominantly in the exosphere, where the mean free path is comparable in length to the pressure scale height. The number of particles able to escape depends on the molecular concentration at the exobase, which is limited by diffusion through the thermosphere.
Three factors strongly contribute to the relative importance of Jeans escape: mass of the molecule, escape velocity of the planet, and heating of the upper atmosphere by radiation from the parent star. Heavier molecules are less likely to escape because they move slower than lighter molecules at the same temperature. This is why hydrogen escapes from an atmosphere more easily than carbon dioxide. Second, a planet with a larger mass tends to have more gravity, so the escape velocity tends to be greater, and fewer particles will gain the energy required to escape. This is why the gas giant planets still retain significant amounts of hydrogen, which escape more readily from Earth's atmosphere. Finally, the distance a planet orbits from a star also plays a part; a close planet has a hotter atmosphere, with higher velocities and hence, a greater likelihood of escape. A distant body has a cooler atmosphere, with lower velocities, and less chance of escape.
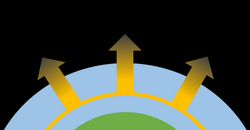
Hydrodynamic escape
An atmosphere with high pressure and temperature can also undergo hydrodynamic escape. In this case, a large amount of thermal energy, usually through extreme ultraviolet radiation, is absorbed by the atmosphere. As molecules are heated, they expand upwards and are further accelerated until they reach escape velocity. In this process, lighter molecules can drag heavier molecules with them through collisions as a larger quantity of gas escapes.[3] Hydrodynamic escape has been observed for exoplanets close to their host star, including the hot Jupiter HD 209458 b.[4]
Non-thermal (suprathermal) escape
Escape can also occur due to non-thermal interactions. Most of these processes occur due to photochemistry or charged particle (ion) interactions.
Photochemical escape
In the upper atmosphere, high energy ultraviolet photons can react more readily with molecules. Photodissociation can break a molecule into smaller components and provide enough energy for those components to escape. Photoionization produces ions, which can get trapped in the planet's magnetosphere or undergo dissociative recombination. In the first case, these ions may undergo escape mechanisms described below. In the second case, the ion recombines with an electron, releases energy, and can escape.[5]
Sputtering escape
Excess kinetic energy from the solar wind can impart sufficient energy to eject atmospheric particles, similar to sputtering from a solid surface. This type of interaction is more pronounced in the absence of a planetary magnetosphere, as the electrically charged solar wind is deflected by magnetic fields, which mitigates the loss of atmosphere.[6]
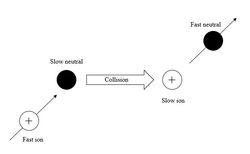
Charge exchange escape
Ions in the solar wind or magnetosphere can charge exchange with molecules in the upper atmosphere. A fast-moving ion can capture the electron from a slow atmospheric neutral, creating a fast neutral and a slow ion. The slow ion is trapped on the magnetic field lines, but the fast neutral can escape.[5]
Polar wind escape
Atmospheric molecules can also escape from the polar regions on a planet with a magnetosphere, due to the polar wind. Near the poles of a magnetosphere, the magnetic field lines are open, allowing a pathway for ions in the atmosphere to exhaust into space. The ambipolar electric field accelerates any ions in the ionosphere, launching along these lines.[8][9]
Impact erosion
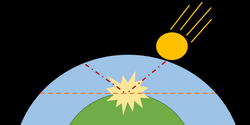
The impact of a large meteoroid can lead to the loss of atmosphere. If a collision is sufficiently energetic, it is possible for ejecta, including atmospheric molecules, to reach escape velocity.[10]
In order to have a significant effect on atmospheric escape, the radius of the impacting body must be larger than the scale height. The projectile can impart momentum, and thereby facilitate escape of the atmosphere, in three main ways: (a) the meteoroid heats and accelerates the gas it encounters as it travels through the atmosphere, (b) solid ejecta from the impact crater heat atmospheric particles through drag as they are ejected, and (c) the impact creates vapor which expands away from the surface. In the first case, the heated gas can escape in a manner similar to hydrodynamic escape, albeit on a more localized scale. Most of the escape from impact erosion occurs due to the third case.[10] The maximum atmosphere that can be ejected is above a plane tangent to the impact site.
Dominant atmospheric escape and loss processes in the Solar System
Earth
Atmospheric escape of hydrogen on Earth is due to charge exchange escape (~60–90%), Jeans escape (~10–40%), and polar wind escape (~10–15%), currently losing about 3 kg/s of hydrogen.[1] The Earth additionally loses approximately 50 g/s of helium primarily through polar wind escape. Escape of other atmospheric constituents is much smaller.[1] A Japanese research team in 2017 found evidence of a small number of oxygen ions on the moon that came from the Earth.[11]
In 1 billion years, the Sun will be 10% brighter, making it hot enough on Earth to dramatically increase the water vapor in the atmosphere where solar ultraviolet light will dissociate H2O, allowing it to gradually escape into space until the oceans dry up.[12]: 159
Venus
Recent models indicate that hydrogen escape on Venus is almost entirely due to suprathermal mechanisms, primarily photochemical reactions and charge exchange with the solar wind. Oxygen escape is dominated by charge exchange and sputtering escape.[13] Venus Express measured the effect of coronal mass ejections on the rate of atmospheric escape of Venus, and researchers found a factor of 1.9 increase in escape rate during periods of increased coronal mass ejections compared with calmer space weather.[14]
Mars
Primordial Mars also suffered from the cumulative effects of multiple small impact erosion events,[15] and recent observations with MAVEN suggest that 66% of the 36Ar in the Martian atmosphere has been lost over the last 4 billion years due to suprathermal escape, and the amount of CO2 lost over the same time period is around 0.5 bar or more.[16]
The MAVEN mission has also explored the current rate of atmospheric escape of Mars. Jeans escape plays an important role in the continued escape of hydrogen on Mars, contributing to a loss rate that varies between 160–1800 g/s.[17] The Jeans escape of hydrogen can be significantly modulated by lower atmospheric processes, such as gravity waves, convection, and dust storms.[18] Oxygen loss is dominated by suprathermal methods: photochemical (~1300 g/s), charge exchange (~130 g/s), and sputtering (~80 g/s) escape combine for a total loss rate of ~1500 g/s. Other heavy atoms, such as carbon and nitrogen, are primarily lost due to photochemical reactions and interactions with the solar wind.[1][13]
Titan and Io
Saturn's moon Titan and Jupiter's moon Io have atmospheres and are subject to atmospheric loss processes. They have no magnetic fields of their own, but orbit planets with powerful magnetic fields, which protects a given moon from the solar wind when its orbit is within the bow shock. However Titan spends roughly half of its orbital period outside of the bow-shock, subjected to unimpeded solar winds. The kinetic energy gained from pick-up and sputtering associated with the solar winds increases thermal escape throughout the orbit of Titan, causing neutral hydrogen to escape.[19] The escaped hydrogen maintains an orbit following in the wake of Titan, creating a neutral hydrogen torus around Saturn. Io, in its orbit around Jupiter, encounters a plasma cloud.[20] Interaction with the plasma cloud induces sputtering, kicking off sodium particles. The interaction produces a stationary banana-shaped charged sodium cloud along a part of the orbit of Io.
Observations of exoplanet atmospheric escape
Studies of exoplanets have measured atmospheric escape as a means of determining atmospheric composition and habitability. The most common method is Lyman-alpha line absorption. Much as exoplanets are discovered using the dimming of a distant star's brightness (transit), looking specifically at wavelengths corresponding to hydrogen absorption describes the amount of hydrogen present in a sphere around the exoplanet.[21] This method indicates that the hot Jupiters HD 209458 b[22] and HD 189733 b[23] and hot Neptune Gliese 436 b[24] are experiencing significant atmospheric escape.
In 2018 it was discovered with the Hubble Space Telescope that atmospheric escape can also be measured with the 1083 nm Helium triplet.[25] This wavelength is much more accessible from ground-based high-resolution spectrographs, when compared to the ultraviolet Lyman-alpha lines. The wavelength around the helium triplet has also the advantage that it is not severely affected by interstellar absorption, which is an issue for Lyman-alpha. Helium has on the other hand the disadvantage that it requires knowledge about the hydrogen-helium ratio to model the mass-loss of the atmosphere. Helium escape was measured around many giant exoplanets, including WASP-107b, WASP-69b, and HD 189733 b. It has also been detected around some mini-Neptunes, such as TOI-560 b[26] and HD 63433 c.[27]
Other atmospheric loss mechanisms
Sequestration is not a form of escape from the planet, but a loss of molecules from the atmosphere and into the planet. It occurs on Earth when water vapor condenses to form rain or glacial ice, when carbon dioxide is sequestered in sediments or cycled through the oceans or when rocks are oxidized (for example, by increasing the oxidation states of ferric rocks from Fe2+ to Fe3+). Gases can also be sequestered by adsorption, where fine particles in the regolith capture gas which adheres to the surface particles.
References
- ↑ 1.0 1.1 1.2 1.3 David C. Catling and Kevin J. Zahnle, The Planetary Air Leak, Scientific American, May 2009, p. 26 (accessed 25 July 2012)
- ↑ Muriel Gargaud, Encyclopedia of Astrobiology, Volume 3, Springer Science & Business Media, May 26, 2011, p. 879.
- ↑ Catling, David C.; Zahnle, Kevin J. (2009). "The Planetary Air Leak". Scientific American 300 (5): 36–43. doi:10.1038/scientificamerican0509-36. ISSN 0036-8733. PMID 19438047. Bibcode: 2009SciAm.300e..36C.
- ↑ Vidal-Madjar, A.; Dsert, J.-M.; Etangs; Hbrard, G.; Ballester, G. E.; Ehrenreich, D.; Ferlet, R.; McConnell, J. C. et al. (2004). "Vidal-Madjar et al., Oxygen and Carbon in HD 209458b". The Astrophysical Journal 604: L69–L72. doi:10.1086/383347.
- ↑ 5.0 5.1 Shematovich, V I; Marov, M Ya (2018-03-31). "Escape of planetary atmospheres: physical processes and numerical models". Physics-Uspekhi 61 (3): 217–246. doi:10.3367/ufne.2017.09.038212. ISSN 1063-7869. Bibcode: 2018PhyU...61..217S.
- ↑ Lundin, Rickard; Lammer, Helmut; Ribas, Ignasi (2007-08-17). "Planetary Magnetic Fields and Solar Forcing: Implications for Atmospheric Evolution". Space Science Reviews 129 (1–3): 245–278. doi:10.1007/s11214-007-9176-4. ISSN 0038-6308. Bibcode: 2007SSRv..129..245L.
- ↑ Goldston, R. J. (1995). Introduction to plasma physics. Rutherford, P. H. (Paul Harding), 1938-. Bristol, UK: Institute of Physics Pub. ISBN 0750303255. OCLC 33079555.
- ↑ Gronoff, G.; Arras, P.; Baraka, S.; Bell, J. M.; Cessateur, G.; Cohen, O.; Curry, S. M.; Drake, J. J. et al. (2020). "Atmospheric Escape Processes and Planetary Atmospheric Evolution" (in en). Journal of Geophysical Research: Space Physics 125 (8). doi:10.1029/2019JA027639. ISSN 2169-9380. https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2019JA027639.
- ↑ "The curious case of Earth's leaking atmosphere". https://phys.org/news/2016-07-curious-case-earth-leaking-atmosphere.html.
- ↑ 10.0 10.1 Ahrens, T J (1993). "Impact Erosion of Terrestrial Planetary Atmospheres". Annual Review of Earth and Planetary Sciences 21 (1): 525–555. doi:10.1146/annurev.ea.21.050193.002521. ISSN 0084-6597. Bibcode: 1993AREPS..21..525A.
- ↑ "Moon's Been Getting Oxygen from Earth's Plants for Billions of Years". 30 January 2017. http://www.space.com/35502-moon-has-oxygen-from-earth-plants.html.
- ↑ Schröder, K.-P.; Connon Smith, Robert (May 1, 2008), "Distant future of the Sun and Earth revisited", Monthly Notices of the Royal Astronomical Society 386 (1): 155–63, doi:10.1111/j.1365-2966.2008.13022.x, Bibcode: 2008MNRAS.386..155S.
- ↑ 13.0 13.1 Lammer, H.; Lichtenegger, H. I. M.; Biernat, H. K.; Erkaev, N. V.; Arshukova, I. L.; Kolb, C.; Gunell, H.; Lukyanov, A. et al. (2006). "Loss of hydrogen and oxygen from the upper atmosphere of Venus". Planetary and Space Science 54 (13–14): 1445–1456. doi:10.1016/j.pss.2006.04.022. Bibcode: 2006P&SS...54.1445L.
- ↑ Edberg, N. J. T.; Nilsson, H.; Futaana, Y.; Stenberg, G.; Lester, M.; Cowley, S. W. H.; Luhmann, J. G.; McEnulty, T. R. et al. (2011). "Atmospheric erosion of Venus during stormy space weather". Journal of Geophysical Research: Space Physics 116 (A9): n/a. doi:10.1029/2011JA016749. ISSN 2156-2202. Bibcode: 2011JGRA..116.9308E.
- ↑ Melosh, H.J.; Vickery, A.M. (April 1989). "Impact erosion of the primordial atmosphere of Mars". Nature 338 (6215): 487–489. doi:10.1038/338487a0. PMID 11536608. Bibcode: 1989Natur.338..487M.
- ↑ Alsaeed, N.; Stone, S.; Yelle, R.; Elrod, M.; Mahaffy, P.; Benna, M.; Slipski, M.; Jakosky, B. M. (2017-03-31). "Mars' atmospheric history derived from upper-atmosphere measurements of 38Ar/36Ar". Science 355 (6332): 1408–1410. doi:10.1126/science.aai7721. ISSN 0036-8075. PMID 28360326. Bibcode: 2017Sci...355.1408J.
- ↑ Jakosky, B. M.; Brain, D.; Chaffin, M.; Curry, S.; Deighan, J.; Grebowsky, J.; Halekas, J.; Leblanc, F. et al. (2018-11-15). "Loss of the Martian atmosphere to space: Present-day loss rates determined from MAVEN observations and integrated loss through time". Icarus 315: 146–157. doi:10.1016/j.icarus.2018.05.030. ISSN 0019-1035. Bibcode: 2018Icar..315..146J.
- ↑ Yiğit, Erdal (2021-12-10). "Martian water escape and internal waves" (in en). Science 374 (6573): 1323–1324. doi:10.1126/science.abg5893. ISSN 0036-8075. PMID 34882460. Bibcode: 2021Sci...374.1323Y. https://www.science.org/doi/10.1126/science.abg5893.
- ↑ Lammer, H.; Stumptner, W.; Bauer, S. J. (1998). "Dynamic escape of H from Titan as consequence of sputtering induced heating". Planetary and Space Science 46 (9–10): 1207–1213. doi:10.1016/S0032-0633(98)00050-6. Bibcode: 1998P&SS...46.1207L.
- ↑ Wilson, J. K.; Mendillo, M.; Baumgardner, J.; Schneider, N. M.; Trauger, J. T.; Flynn, B. (2002). "The dual sources of Io's sodium clouds". Icarus 157 (2): 476–489. doi:10.1006/icar.2002.6821. Bibcode: 2002Icar..157..476W.
- ↑ Owen, James E. (2019-05-30). "Atmospheric Escape and the Evolution of Close-In Exoplanets". Annual Review of Earth and Planetary Sciences 47 (1): 67–90. doi:10.1146/annurev-earth-053018-060246. ISSN 0084-6597. Bibcode: 2019AREPS..47...67O.
- ↑ Vidal-Madjar, A.; des Etangs, A. Lecavelier; Désert, J.-M.; Ballester, G. E.; Ferlet, R.; Hébrard, G.; Mayor, M. (March 2003). "An extended upper atmosphere around the extrasolar planet HD209458b". Nature 422 (6928): 143–146. doi:10.1038/nature01448. ISSN 0028-0836. PMID 12634780. Bibcode: 2003Natur.422..143V.
- ↑ Lecavelier des Etangs, A.; Ehrenreich, D.; Vidal-Madjar, A.; Ballester, G. E.; Désert, J.-M.; Ferlet, R.; Hébrard, G.; Sing, D. K. et al. (May 2010). "Evaporation of the planet HD 189733b observed in H I Lyman- α". Astronomy and Astrophysics 514: A72. doi:10.1051/0004-6361/200913347. ISSN 0004-6361. Bibcode: 2010A&A...514A..72L.
- ↑ Ehrenreich, David; Bourrier, Vincent; Wheatley, Peter J.; des Etangs, Alain Lecavelier; Hébrard, Guillaume; Udry, Stéphane; Bonfils, Xavier; Delfosse, Xavier et al. (June 2015). "A giant comet-like cloud of hydrogen escaping the warm Neptune-mass exoplanet GJ 436b". Nature 522 (7557): 459–461. doi:10.1038/nature14501. ISSN 0028-0836. PMID 26108854. Bibcode: 2015Natur.522..459E.
- ↑ Spake, J. J.; Sing, D. K.; Evans, T. M.; Oklopčić; A.; Bourrier, V.; Kreidberg, L.; Rackham, B. V. et al. (2018-05-01). "Helium in the eroding atmosphere of an exoplanet". Nature 557 (7703): 68–70. doi:10.1038/s41586-018-0067-5. ISSN 0028-0836. PMID 29720632. Bibcode: 2018Natur.557...68S. https://ui.adsabs.harvard.edu/abs/2018Natur.557...68S.
- ↑ Zhang, Michael; Knutson, Heather A.; Dai, Fei; Wang, Lile; Ricker, George R.; Schwarz, Richard P.; Mann, Christopher; Collins, Karen (2022-07-01). "Detection of Atmospheric Escape from Four Young Mini Neptunes". The Astronomical Journal 165 (2): 62. doi:10.3847/1538-3881/aca75b. Bibcode: 2023AJ....165...62Z. https://ui.adsabs.harvard.edu/abs/2022arXiv220713099Z.
- ↑ Zhang, Michael; Knutson, Heather A.; Wang, Lile; Dai, Fei; dos Santos, Leonardo A.; Fossati, Luca; Henry, Gregory W.; Ehrenreich, David et al. (2022-01-17). "Detection of Ongoing Mass Loss from HD 63433c, a Young Mini-Neptune". The Astronomical Journal 163 (2): 68. doi:10.3847/1538-3881/ac3f3b. ISSN 0004-6256. Bibcode: 2022AJ....163...68Z.
Further reading
- Zahnle, Kevin J.; Catling, David C. (May 2009). "Our Planet's Leaky Atmosphere". Scientific American. http://www.scientificamerican.com/article.cfm?id=how-planets-lose-their-atmospheres.
- Ingersoll, Andrew P. (2013). Planetary climates. Princeton, N.J.: Princeton University Press. ISBN 9781400848232. OCLC 855906548.
- Hunten, D. M. (1993). "Atmospheric evolution of the terrestrial planets". Science 259 (5097): 915–920. doi:10.1126/science.259.5097.915. Bibcode: 1993Sci...259..915H.
- Lammer, H.; Bauer, S. J. (1993). "Atmospheric mass-loss from Titan by sputtering". Planetary and Space Science 41 (9): 657–663. doi:10.1016/0032-0633(93)90049-8. Bibcode: 1993P&SS...41..657L.
 |
