Medicine:Allan–Herndon–Dudley syndrome
| Allan–Herndon–Dudley syndrome | |
|---|---|
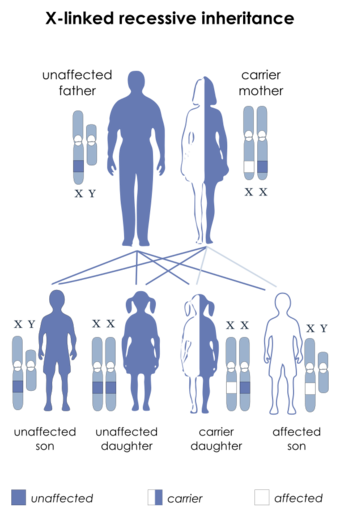 | |
| This condition is inherited in an X-linked recessive manner |
Allan–Herndon–Dudley syndrome is a rare X-linked inherited disorder of brain development that causes both moderate to severe intellectual disability and problems with speech and movement.[1]
Allan–Herndon–Dudley syndrome, which is named eponymously for William Allan, Florence C. Dudley, and C. Nash Herndon,[2][3] results from a mutation of the thyroid hormone transporter MCT8 (also referred to as SLC16A2). Consequently, thyroid hormones are unable to enter the nervous system, which depends on thyroid signaling for proper function and development.[citation needed]
Signs and symptoms
It is estimated that 80–99% of people with Allan–Herndon–Dudley syndrome will have biparietal narrowing (narrowing of skull), ataxia, abnormalities of the neck, and both absent speech development and aphasia. Weak muscle tone (hypotonia) and underdevelopment of many muscles (muscle hypoplasia) are common in children with Allan–Herndon–Dudley syndrome. Development of joint deformities called contractures, which restrict the movement of certain joints, are common as people age. Mobility is further limited by abnormal muscle stiffness (spasticity), muscle weakness, and involuntary movements of the arms and legs. Many people with Allan–Herndon–Dudley syndrome are unable to walk independently and become wheelchair-reliant by adulthood.[4]
Endocrine phenotype
The typical hormonal signature of AHDS is marked by low free T4 and normal or elevated free T3 concentration, which translates to increased calculated deiodinase activity (SPINA-GD).[5][6][7]
Genetics
This condition is inherited in an X-linked recessive pattern. A condition is considered X-linked if the mutated gene that causes the disorder is located on the X chromosome, one of the two sex chromosomes. In males (who have only one X chromosome), one altered copy of the gene in each cell is sufficient to cause the condition. In females (who have two X chromosomes), a mutation must be present in both copies of the gene to cause the disorder. Males are affected by X-linked recessive disorders much more frequently than females. A striking characteristic of X-linked inheritance is that fathers cannot pass X-linked traits to their sons.[citation needed]
In X-linked recessive inheritance, a female with one altered copy of the gene in each cell is called a carrier. She can pass on the mutated gene, but usually does not experience signs and symptoms of the disorder. Carriers of SLC16A2 mutations have normal intelligence and do not experience problems with movement. Some carriers have been diagnosed with thyroid disease, a condition which is relatively common in the general population. It is unclear whether thyroid disease is related to SLC16A2 mutations in these cases.[citation needed]
Pathogenesis
Mutations in the SLC16A2 gene cause Allan–Herndon–Dudley syndrome. The SLC16A2 gene, also known as MCT8, provides instructions for making a protein that plays a critical role in the development of the nervous system. This protein transports a particular hormone into nerve cells in the developing brain. This hormone, called triiodothyronine or T3, is produced by the thyroid. T3 appears to be critical for the normal formation and growth of nerve cells, as well as the development of junctions between nerve cells (synapses) where cell-to-cell communication occurs. T3 and other forms of thyroid hormone also help regulate the development of other organs and control the rate of chemical reactions in the body.[citation needed]
Gene mutations alter the structure and function of the SLC16A2 protein. As a result, this protein is unable to transport T3 into nerve cells effectively. A lack of this critical hormone in certain parts of the brain disrupts normal brain development, resulting in intellectual disability and problems with movement. Excess amounts of T3 circulate in the bloodstream. It is unclear if this is a consequence of compensatory hyperdeiodination or if it results from impaired uptake by certain cell types. Increased T3 levels in the blood may be toxic to some organs and contribute to the signs and symptoms of Allan–Herndon–Dudley syndrome.[citation needed]
The signature of low T4 and high T3 was for a long time assumed to be caused by either compensatory hyperdeiodination or impaired uptake of T3 in target tissues. A third hypotheses suggested it to ensue from impaired outward transport of thyroxine from thyroid cells and subsequently substrate-mediated overactivity of intrathyroidal deiodinases.[8] This latter hypothesis is supported by results of in silico experiments with computer simulations.[9]
Several studies have documented the potentially dangerous effects of the silymarin mixture on the MCT8 transporter. All of the flavonolignan compounds found in the silymarin mixture seem to block the uptake of thyroid hormones into the cells by selectively blocking the MCT8 transmembrane transporter.[10] The authors of several studies noted that especially silychristin, one of the compounds of the silymarin mixture seems to be perhaps the most powerful and selective inhibitor for the MCT8 transporter. Due to the essential role played by the thyroid hormone in human metabolism in general it is believed that the intake of silymarin can lead to disruptions of the thyroid system. Because the thyroid hormones and the MCT8 as well are known to play a critical role during early and fetal development, the administration of silymarin during pregnancy is especially thought to be dangerous, potentially leading to the Allan–Herndon–Dudley syndrome.[citation needed]
Treatment
In May 2013, the US FDA granted Orphan drug status to Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. This was following the use of DITPA towards a child in Australia, under compassionate grounds.[11]
Theoretical considerations suggested TRIAC (triiodothyroacetate or tiratricol, a natural non-classical thyroid hormone) to be beneficial. In 2014, a case was demonstrated in which therapy with TRIAC in early childhood led to significant improvement of cognition and mobility.[12] A first clinical trial[13] demonstrated TRIAC to be safe and effective.[14]
References
- ↑ "Allan-Herndon-Dudley syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program" (in en). https://rarediseases.info.nih.gov/diseases/5617/allan-herndon-dudley-syndrome.
- ↑ synd/1438 at Who Named It?
- ↑ Allan, William; Herndon, C. N.; Dudley, Florence C. (1944). "Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly". American Journal of Mental Deficiency 48: 325–34.
- ↑ "Allan-Herndon-Dudley syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program" (in en). https://rarediseases.info.nih.gov/diseases/5617/allan-herndon-dudley-syndrome.
- ↑ Schwartz, CE; Stevenson, RE (June 2007). "The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome.". Best Practice & Research. Clinical Endocrinology & Metabolism 21 (2): 307–21. doi:10.1016/j.beem.2007.03.009. PMID 17574010.
- ↑ van Geest, FS; Groeneweg, S; Visser, WE (March 2021). "Monocarboxylate transporter 8 deficiency: update on clinical characteristics and treatment.". Endocrine 71 (3): 689–695. doi:10.1007/s12020-020-02603-y. PMID 33650046.
- ↑ Schupper, A; Barash, G; Benyamini, L; Ben-Haim, R; Heyman, E; Lahat, E; Bassan, H (May 2023). "Simple Evaluation of Thyroid Function Leading to the Diagnosis of Allan-Herndon-Dudley Syndrome, a Rare Neurodevelopmental Disorder.". The Israel Medical Association Journal 25 (5): 374–376. PMID 37245112.
- ↑ Müller, J; Heuer, H (July 2012). "Understanding the hypothalamus-pituitary-thyroid axis in mct8 deficiency.". European Thyroid Journal 1 (2): 72–9. doi:10.1159/000339474. PMID 24783000.
- ↑ Wolff, TM; Veil, C; Dietrich, JW; Müller, MA (2022). "Mathematical modeling and simulation of thyroid homeostasis: Implications for the Allan-Herndon-Dudley syndrome.". Frontiers in Endocrinology 13: 882788. doi:10.3389/fendo.2022.882788. PMID 36568087.
- ↑ Johannes, Jörg; Jayarama-Naidu, Roopa; Meyer, Franziska; Wirth, Eva Katrin; Schweizer, Ulrich; Schomburg, Lutz; Köhrle, Josef; Renko, Kostja (24 February 2016). "Silychristin, a Flavonolignan Derived From the Milk Thistle, Is a Potent Inhibitor of the Thyroid Hormone Transporter MCT8". Endocrinology (The Endocrine Society) 157 (4): 1694–1701. doi:10.1210/en.2015-1933. ISSN 0013-7227. PMID 26910310.
- ↑ Verge, Charles F.; Konrad, Daniel; Cohen, Michal; Di Cosmo, Caterina; Dumitrescu, Alexandra M.; Marcinkowski, Teresa; Hameed, Shihab; Hamilton, Jill et al. (2012). "Diiodothyropropionic Acid (DITPA) in the Treatment of MCT8 Deficiency". The Journal of Clinical Endocrinology & Metabolism 97 (12): 4515–23. doi:10.1210/jc.2012-2556. PMID 22993035.
- ↑ Iglesias, Ainhoa; Palomares, María; Morte, Beatriz; Obregón, María Jesús; Bernal, Juan (September 10, 2014). "TRIAC treatment of an infant with Allan-Herndon-Dudley Syndrome (AHDS): Effects on iodothyronines in serum and cerebrospinal fluid". 38th Annual Meeting of the European Thyroid Association. Santiago de Compostela.
- ↑ Clinical trial number NCT02060474 for "Triac Trial in MCT8 Patients" at ClinicalTrials.gov
- ↑ Groeneweg, S; Peeters, RP; Moran, C; Stoupa, A; Auriol, F; Tonduti, D; Dica, A; Paone, L et al. (September 2019). "Effectiveness and safety of the tri-iodothyronine analogue Triac in children and adults with MCT8 deficiency: an international, single-arm, open-label, phase 2 trial.". The Lancet. Diabetes & Endocrinology 7 (9): 695–706. doi:10.1016/S2213-8587(19)30155-X. PMID 31377265.
External links
- GeneReviews/NCBI/NIH/UW entry on MCT8 (SLC16A2)-Specific Thyroid Hormone Cell Transporter Deficiency
- Allan–Herndon–Dudley syndrome at National Library of Medicine
| Classification |
|---|
 |

