Biology:Pendrin
Pendrin is an anion exchange protein that in humans is encoded by the SLC26A4 gene (solute carrier family 26, member 4).[1][2] Pendrin was initially identified as a sodium-independent chloride-iodide exchanger[3] with subsequent studies showing that it also accepts formate and bicarbonate as substrates.[4][5] Pendrin is similar to the Band 3 transport protein found in red blood cells. Pendrin is the protein which is mutated in Pendred syndrome, which is an autosomal recessive disorder characterized by sensorineural hearing loss, goiter and a partial organification problem detectable by a positive perchlorate test.[6]
Pendrin orthologs are responsible for mediating the electroneutral exchange of chloride (Cl−) for bicarbonate (HCO3−) across a plasma membrane in the chloride cells of freshwater fish,[7] and show changes in expression in response to salinity change in the gills of Atlantic stingrays.[8]
By phylogenetic analysis, pendrin has been found to be a close relative of prestin present on the hair cells or organ of corti in the inner ear. Prestin is primarily an electromechanical transducer but pendrin is an ion transporter.
Function
Pendrin is an ion exchanger found in many types of cells in the body. High levels of pendrin expression have been identified in the inner ear and thyroid.[9]
Thyroid
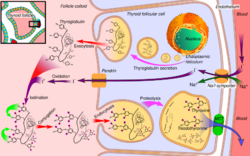
In the thyroid, pendrin is expressed by thyroid follicular cells. Na+/I− symporter imports iodide (I−) into the cell across its basolateral side, and pendrin extrudes the I− across the cell's apical membrane into the thyroid colloid.[10]
Inner ear
The exact function of pendrin in the inner ear remains unclear; however, pendrin may play a role in acid-base balance as a chloride-bicarbonate exchanger, regulate volume homeostasis through its ability to function as a chloride-formate exchanger[11][12] or indirectly modulate the calcium concentration of the endolymph.[13] Pendrin is also expressed in the kidney, and has been localized to the apical membrane of a population of intercalated cells in the cortical collecting duct where it is involved in bicarbonate secretion.[14][15]
Kidney
Renal β-intercalated cells of the late distal tube and collecting duct express pendrin upon their apical membrane, resorbing one Cl− in exchange for secreting a HCO3−, with Cl− subsequently extruded from the cell by a basolateral Cl− channel. β-intercalated cells thus utilise pendrin to contribute to acid-base homeostasis by excreting base (HCO3−) into urine. Additionally, β-intercalated cells may use pendrin in concert with a Na+/HCO3−/2Cl− antiporter in order to resorb NaCl.[16]
Clinical significance
Mutations in this gene are associated with Pendred syndrome, the most common form of syndromic deafness, an autosomal-recessive disease. Pendred syndrome is characterized by thyroid goiter and enlargement of the vestibular aqueduct resulting in deafness; however, despite being expressed in the kidney, individuals with Pendred syndrome do not show any kidney-related acid-base, or volume abnormalities under basal conditions. This is probably the result of other bicarbonate or chloride transporters in the kidney compensating for any loss of pendrin function. Only under extreme situations of salt depletion or metabolic alkalosis, or with inactivation of the sodium-chloride cotransporter, are fluid and electrolyte disorders manifested in these patients.[17] SLC26A4 is highly homologous to the SLC26A3 gene; they have similar genomic structures and this gene is located 3' of the SLC26A3 gene. The encoded protein has homology to sulfate transporters.[1]
Another little-understood role of pendrin is in airway hyperreactivity and inflammation, as during asthma attacks and allergic reactions. Expression of pendrin in the lung increases in response to allergens and high concentrations of IL-13,[18][19] and overexpression of pendrin results in airway inflammation, hyperreactivity, and increased mucus production.[20][21] These symptoms could result from pendrin's effects on ion concentration in the airway surface liquid, possibly causing the liquid to be less hydrated.[22]
References
- ↑ 1.0 1.1 "Entrez Gene: SLC26A4 solute carrier family 26, member 4". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5172.
- ↑ "Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS)". Nature Genetics 17 (4): 411–422. December 1997. doi:10.1038/ng1297-411. PMID 9398842. https://zenodo.org/record/1233411.
- ↑ "The Pendred syndrome gene encodes a chloride-iodide transport protein". Nature Genetics 21 (4): 440–443. April 1999. doi:10.1038/7783. PMID 10192399.
- ↑ "Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange". American Journal of Physiology. Cell Physiology 278 (1): C207–C211. January 2000. doi:10.1152/ajpcell.2000.278.1.c207. PMID 10644529.
- ↑ "Pendrin: an apical Cl-/OH-/HCO3- exchanger in the kidney cortex". American Journal of Physiology. Renal Physiology 280 (2): F356–F364. February 2001. doi:10.1152/ajprenal.2001.280.2.f356. PMID 11208611.
- ↑ Principles of molecular medicine. Totowa, NJ: Humana Press. 2006. p. 957. ISBN 1-58829-202-9. https://books.google.com/books?id=j-_rAKRf3WwC&q=prestin+pendrin&pg=PA347.
- ↑ "Osmoregulation in Zebrafish: Ion Transport Mechanisms and Functional Regulation". EXCLI Journal 14: 627-659. 2015. doi:10.17179/excli2015-246. PMID 26600749.
- ↑ "Pendrin immunoreactivity in the gill epithelium of a euryhaline elasmobranch". American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 283 (4): R983-R992. 2002. doi:10.1152/ajpregu.00178.2002. PMID 12228069.
- ↑ "Controversies concerning the role of pendrin as an apical iodide transporter in thyroid follicular cells". Cellular Physiology and Biochemistry 28 (3): 485–490. 2011-01-01. doi:10.1159/000335103. PMID 22116361.
- ↑ "Factors influencing the study of peroxidase-generated iodine species and implications for thyroglobulin synthesis". Thyroid 18 (7): 769–774. July 2008. doi:10.1089/thy.2007.0310. PMID 18631006.
- ↑ "Chloride/formate exchange with formic acid recycling: a mechanism of active chloride transport across epithelial membranes". Proceedings of the National Academy of Sciences of the United States of America 82 (18): 6362–6365. September 1985. doi:10.1073/pnas.82.18.6362. PMID 3862136. Bibcode: 1985PNAS...82.6362K.
- ↑ "Failure of fluid absorption in the endolymphatic sac initiates cochlear enlargement that leads to deafness in mice lacking pendrin expression". PLOS ONE 5 (11): e14041. November 2010. doi:10.1371/journal.pone.0014041. PMID 21103348. Bibcode: 2010PLoSO...514041K.
- ↑ "Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model". American Journal of Physiology. Renal Physiology 292 (5): F1345–F1353. May 2007. doi:10.1152/ajprenal.00487.2006. PMID 17299139.
- ↑ "The Renal Physiology of Pendrin (SLC26A4) and Its Role in Hypertension". Epithelial Anion Transport in Health and Disease: The Role of the SLC26 Transporters Family. Novartis Foundation Symposia. 273. 2006. pp. 231–9; discussion 239–43, 261–4. doi:10.1002/0470029579.ch15. ISBN 978-0-470-02957-2.
- ↑ "Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion". Proceedings of the National Academy of Sciences of the United States of America 98 (7): 4221–4226. March 2001. doi:10.1073/pnas.071516798. PMID 11274445. Bibcode: 2001PNAS...98.4221R.
- ↑ Berne & Levy Physiology (8th ed.). Philadelphia, PA: Elsevier. 2024. ISBN 978-0-323-84790-2.
- ↑ "Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome". Clinical Nephrology 69 (6): 450–453. June 2008. doi:10.5414/cnp69450. PMID 18538122.
- ↑ "Dissecting asthma using focused transgenic modeling and functional genomics". The Journal of Allergy and Clinical Immunology 116 (2): 305–311. August 2005. doi:10.1016/j.jaci.2005.03.024. PMID 16083784.
- ↑ "IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production". American Journal of Respiratory Cell and Molecular Biology 36 (2): 244–253. February 2007. doi:10.1165/rcmb.2006-0180OC. PMID 16980555.
- ↑ "Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels". Journal of Immunology 178 (8): 5144–5153. April 2007. doi:10.4049/jimmunol.178.8.5144. PMID 17404297.
- ↑ "Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease". Journal of Immunology 180 (9): 6262–6269. May 2008. doi:10.4049/jimmunol.180.9.6262. PMID 18424749.
- ↑ "The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model". Journal of Immunology 181 (3): 2203–2210. August 2008. doi:10.4049/jimmunol.181.3.2203. PMID 18641360.
Further reading
- "Physiological roles and regulation of mammalian sulfate transporters". Physiological Reviews 81 (4): 1499–1533. October 2001. doi:10.1152/physrev.2001.81.4.1499. PMID 11581495.
- "Linkage of congenital, recessive deafness (DFNB4) to chromosome 7q31 and evidence for genetic heterogeneity in the Middle Eastern Druze population". Human Molecular Genetics 4 (9): 1637–1642. September 1995. doi:10.1093/hmg/4.9.1637. PMID 8541853.
- "Pendred syndrome (goitre and sensorineural hearing loss) maps to chromosome 7 in the region containing the nonsyndromic deafness gene DFNB4". Nature Genetics 12 (4): 421–423. April 1996. doi:10.1038/ng0496-421. PMID 8630497.
- "Pendred syndrome maps to chromosome 7q21-34 and is caused by an intrinsic defect in thyroid iodine organification". Nature Genetics 12 (4): 424–426. April 1996. doi:10.1038/ng0496-424. PMID 8630498.
- "Thyroid peroxidase: evidence for disease gene exclusion in Pendred's syndrome". Clinical Endocrinology 44 (4): 441–446. April 1996. doi:10.1046/j.1365-2265.1996.714536.x. PMID 8706311.
- "The gene for Pendred syndrome is located between D7S501 and D7S692 in a 1.7-cM region on chromosome 7q". Genomics 40 (1): 48–54. February 1997. doi:10.1006/geno.1996.4541. PMID 9070918.
- "A mutation in PDS causes non-syndromic recessive deafness". Nature Genetics 18 (3): 215–217. March 1998. doi:10.1038/ng0398-215. PMID 9500541. https://zenodo.org/record/1233379.
- "Two frequent missense mutations in Pendred syndrome". Human Molecular Genetics 7 (7): 1099–1104. July 1998. doi:10.1093/hmg/7.7.1099. PMID 9618166.
- "Molecular analysis of the PDS gene in Pendred syndrome". Human Molecular Genetics 7 (7): 1105–1112. July 1998. doi:10.1093/hmg/7.7.1105. PMID 9618167.
- "Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations". Human Genetics 104 (2): 188–192. February 1999. doi:10.1007/s004390050933. PMID 10190331.
- "Pendred syndrome: phenotypic variability in two families carrying the same PDS missense mutation". American Journal of Medical Genetics 90 (1): 38–44. January 2000. doi:10.1002/(SICI)1096-8628(20000103)90:1<38::AID-AJMG8>3.0.CO;2-R. PMID 10602116.
- "Enlarged vestibular aqueduct: a radiological marker of pendred syndrome, and mutation of the PDS gene". QJM 93 (2): 99–104. February 2000. doi:10.1093/qjmed/93.2.99. PMID 10700480.
- "A novel mutation in the pendrin gene associated with Pendred's syndrome". Clinical Endocrinology 52 (3): 279–285. March 2000. doi:10.1046/j.1365-2265.2000.00930.x. PMID 10718825.
- "Expression of pendrin and the Pendred syndrome (PDS) gene in human thyroid tissues". The Journal of Clinical Endocrinology and Metabolism 85 (5): 2028–2033. May 2000. doi:10.1210/jcem.85.5.6519. PMID 10843192.
- "Deafness heterogeneity in a Druze isolate from the Middle East: novel OTOF and PDS mutations, low prevalence of GJB2 35delG mutation and indication for a new DFNB locus". European Journal of Human Genetics 8 (6): 437–442. June 2000. doi:10.1038/sj.ejhg.5200489. PMID 10878664.
- "Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger". Genomics 70 (1): 102–112. November 2000. doi:10.1006/geno.2000.6355. PMID 11087667.
- "Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations". Human Mutation 17 (5): 403–411. May 2001. doi:10.1002/humu.1116. PMID 11317356.
External links
- GeneReviews/NCBI/NIH/UW entry on Pendred Syndrome/DFNB4
- Description at oto.wustl.edu
- SLC26A4+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
Template:Thyroid hormone metabolism enzymes and transporters
 |
