Medicine:Apparent mineralocorticoid excess syndrome
| Apparent mineralocorticoid excess syndrome | |
|---|---|
| Other names | AME, 11-beta-hydroxysteroid dehydrogenase deficiency type 2, Ulick syndrome. |
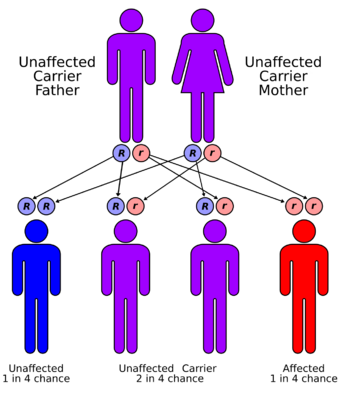 | |
| Apparent mineralocorticoid excess syndrome has an autosomal recessive pattern of inheritance | |
| Symptoms | Hypertension, hypokalemia, metabolic alkalosis, and low plasma renin activity.[1] |
Apparent mineralocorticoid excess is an autosomal recessive[2] disorder causing hypertension (high blood pressure), hypernatremia (increased blood sodium concentration) and hypokalemia (decreased blood potassium concentration). It results from mutations in the HSD11B2 gene, which encodes the kidney isozyme of 11β-hydroxysteroid dehydrogenase type 2. In an unaffected individual, this isozyme inactivates circulating cortisol to the less active metabolite cortisone. The inactivating mutation leads to elevated local concentrations of cortisol in the aldosterone sensitive tissues like the kidney. Cortisol at high concentrations can cross-react and activate the mineralocorticoid receptor due to the non-selectivity of the receptor, leading to aldosterone-like effects in the kidney. This is what causes the hypokalemia, hypertension, and hypernatremia associated with the syndrome. Patients often present with severe hypertension and end-organ changes associated with it like left ventricular hypertrophy, retinal, renal and neurological vascular changes along with growth retardation and failure to thrive. In serum both aldosterone and renin levels are low.[3]
Signs and symptoms
This disorder presents similarly to hyperaldosteronism, leading to feedback inhibition of aldosterone. Common symptoms include hypertension, hypokalemia, metabolic alkalosis, and low plasma renin activity.[1]
DOC excess syndrome is an excessive secretion of 21-hydroxyprogesterone also called 11-Deoxycorticosterone from adrenal glands and may cause mineralocorticoid hypertension.[4][5][6]
Genetics
AME is inherited in an autosomal recessive manner.[2] This means the defective gene responsible for the disorder is located on an autosome, and two copies of the defective gene (one inherited from each parent) are required in order to be born with the disorder. The parents of an individual with an autosomal recessive disorder both carry one copy of the defective gene, but usually do not experience any signs or symptoms of the disorder.[7]
Diagnosis
Other conditions such as Liddle's Syndrome can mimic the clinical features of AME, so diagnosis can be made by calculating the ratio of free urinary cortisol to free urinary cortisone. Since AME patients create less cortisone, the ratio will much be higher than non-affected patients.[8] Alternatively, one could differentiate between the two syndromes by administering a potassium-sparing diuretic. Patients with Liddle's syndrome will only respond to a diuretic that binds the ENaC channel, whereas those with AME will respond to a diuretic that binds to ENaC or the mineralcorticoid receptor.[9]
Treatment
The treatment for AME is based on the blood pressure control with Aldosterone antagonist like Spironolactone which also reverses the hypokalemic metabolic alkalosis and other anti-hypertensives. Renal transplant is found curative in almost all clinical cases.[10] AME is exceedingly rare, with fewer than 100 cases recorded worldwide.[8]
History
Apparent mineralocorticoid excess is a rare form of monogenic hypertension that is transmitted as an autosomal recessive trait. The clinical symptoms of AME were first reported in 1974 by a Professor from Switzerland; Edmond A Werder in a 3-year-old girl with low birth weight, delayed growth, polydipsia, polyuria, and hypertension. In 1977, the US Professor Maria New identified patients with similar symptoms, characterized their biochemical profiles, and named the disease AME. Initially, it was speculated that HSD11B1 (encoding 11β-hydroxysteroid dehydrogenase type 1 [11β-HSD1]) was the causative gene but no mutation was detected in AME patients; thus, the focus was shifted to other candidate genes. In 1995, the US Professor Robert Wilson identified the first HSD11B2 mutation in several siblings with typical characteristics of AME from a consanguineous Iranian family, unraveling the genetic defects of AME. The molecular pathogenesis of AME primarily results from a deficiency in the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which is involved in the peripheral metabolism of cortisol. In 1999, B. Scott Nunez; another US professor, summarized the AME genotype–phenotype correlation by studying 14 affected children and proposed that clinical and/or biochemical parameters and enzyme activity were closely related [11]
Liquorice effect
Liquorice consumption may also cause a temporary form of AME due to its ability to block 11β-hydroxysteroid dehydrogenase type 2, in turn causing increased levels of cortisol.[12][13] Cessation of licorice consumption will reverse this form of AME.[14]
See also
- Inborn errors of steroid metabolism
- 11β-Hydroxylase I deficiency
- Hyperaldosteronism
- Pseudohyperaldosteronism
- Glucocorticoid-remediable aldosteronism
- Aldosterone and aldosterone synthase
- Maria New
References
- ↑ 1.0 1.1 Palermo, Mario; Quinkler, Marcus; Stewart, Paul M. (2004). "Apparent mineralocorticoid excess syndrome: an overview". Arquivos Brasileiros de Endocrinologia & Metabologia (FapUNIFESP (SciELO)) 48 (5): 687–696. doi:10.1590/s0004-27302004000500015. ISSN 0004-2730. PMID 15761540.
- ↑ 2.0 2.1 Levtchenko, E. N.; Deinum, J.; Knoers, N. V. a. M.; Hermus, A. R.; Monnens, L. a. H.; Lenders, J. W. M. (2007). "Van gen naar ziekte; 'apparent mineralocorticoid excess'-syndroom, een syndroom met ogenschijnlijke overmaat aan mineralocorticoïden" (in Dutch). Nederlands Tijdschrift voor Geneeskunde 151 (12): 692–694. PMID 17447595.
- ↑ Al-Harbi, Taiba; Al-Shaikh, Adnan (1 January 2012). "Apparent mineralocorticoid excess syndrome: report of one family with three affected children". Journal of Pediatric Endocrinology and Metabolism 25 (11–12): 1083–8. doi:10.1515/jpem-2012-0113. PMID 23329753.
- ↑ Marques, Pedro; Tufton, Nicola; Bhattacharya, Satya; Caulfield, Mark; Akker, Scott A (3 May 2019). "Hypertension due to a deoxycorticosterone-secreting adrenal tumour diagnosed during pregnancy". Endocrinology, Diabetes & Metabolism Case Reports 2019. doi:10.1530/EDM-18-0164. PMID 31051469.
- ↑ Gupta, Saurabh; Melendez, Jose; Khanna, Apurv (2010). "Deoxycorticosterone Producing Tumor as a Cause of Resistant Hypertension". Case Reports in Medicine 2010: 372719. doi:10.1155/2010/372719. PMID 20671982.
- ↑ Deoxycorticosterone (DOC) at eMedicine
- ↑ "Autosomal recessive: MedlinePlus Medical Encyclopedia". https://medlineplus.gov/ency/article/002052.htm.
- ↑ 8.0 8.1 "Apparent mineralocorticoid excess syndrome: an overview.". Arq Bras Endocrinol Metabol 48 (5): 687–696. Oct 2004. doi:10.1590/S0004-27302004000500015. PMID 15761540. http://eprints.uniss.it/257/1/Palermo_M_Articolo_2004_Apparent.pdf.
- ↑ DG, Warnock (2001). "Liddle's syndrome: genetics and mechanisms of Na+ channel defects". Contributions to Nephrology (Contrib Nephrol) (136): 1–10. ISSN 0302-5144. PMID 11688373. https://pubmed.ncbi.nlm.nih.gov/11688373/. Retrieved November 10, 2023.
- ↑ Marshall, Ian; New, Maria I. (2003). "Mineralocorticoid Disorders, Genetic Basis of". Encyclopedia of Hormones. Elsevier. pp. 679–685. doi:10.1016/b0-12-341103-3/00105-4. ISBN 9780123411037.
- ↑ Zhang, Lu, Yt. (2022). "Apparent mineralocorticoid excess: comprehensive overview of molecular genetics". Translational Medicine 2022 20: 500. doi:10.1186/s12967-022-03698-9.
- ↑ "HSD11B2 Gene". https://www.genecards.org/cgi-bin/carddisp.pl?gene=HSD11B2.
- ↑ Sontia, Bruno; Mooney, Jan; Gaudet, Lise; Touyz, Rhian M. (February 2008). "Pseudohyperaldosteronism, Liquorice, and Hypertension". The Journal of Clinical Hypertension 10 (2): 153–157. doi:10.1111/j.1751-7176.2008.07470.x. PMID 18256580.
- ↑ Gallacher, Stuart Declan; Tsokolas, Georgios; Dimitropoulos, Ioannis (2017). "Liquorice-induced apparent mineralocorticoid excess presenting in the emergency department". Clinical Medicine (Royal College of Physicians) 17 (1): 43–45. doi:10.7861/clinmedicine.17-1-43. ISSN 1470-2118. PMID 28148579.
External links
- Apparent mineralocorticoid excess at NIH's Office of Rare Diseases
| Classification | |
|---|---|
| External resources |
 |

