Medicine:Beck–Fahrner syndrome
| Beck–Fahrner syndrome | |
|---|---|
| Other names | BEFAHRS, TET3 deficiency |
 | |
| Specialty | Medical genetics, pediatrics |
| Symptoms | global developmental delay, psychomotor retardation, neurodevelopmental disorders, psychiatric disorders, hypotonia, epilepsy, dysmorphic features, strabismus, hearing loss, congenital heart defects |
| Usual onset | Present at birth |
| Duration | Lifelong |
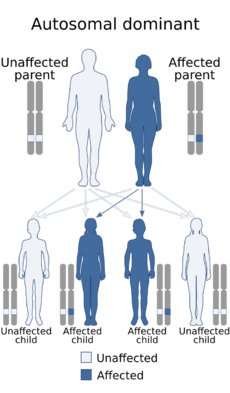
| Types | de novo, autosomal dominant |
| Causes | Mutations of the TET3 gene |
| Diagnostic method | molecular diagnostics, genetic testing |
| Treatment | physical therapy, occupational therapy, educational therapy, speech therapy, assistive technology, anticonvulsant |
Beck–Fahrner syndrome, also known as BEFAHRS and TET3 deficiency, is a rare genetic disorder caused by mutations of the TET3 gene. It can occur de novo or can be inherited in an autosomal dominant manner. Mutations in the TET3 gene disrupts DNA demethylation—an essential epigenetic mechanism—during early embryogenesis and development of the nervous system. Most common clinical presentation includes global developmental delay, psychomotor retardation, neurodevelopmental disorders, hypotonia, epilepsy and dysmorphic features. Diagnosis involves molecular and genetic testing in the context of typical symptoms. Management is supportive, aimed at improving quality of life.
Signs and symptoms
Beck–Fahrner syndrome, also referred to as "BEFAHRS", is characterized by a mnemonic encompassing its prominent features: behavioral differences, epilepsy, facial features, autistic features, hypotonia, retardation of psychomotor development, and size differences.[1]
The most common neurodevelopmental symptoms associated with Beck–Fahrner syndrome include a delay in global development, decreased tone of muscles, slow progress in mental and physical activities, delayed speech, and difficulties with fine and gross motor skills.[2] Intellectual and learning disabilities are commonly present,[3] and more than two-thirds of affected individuals have autism spectrum disorder or social communication disorder.[4] Additionally, attention deficit hyperactivity disorder, obsessive–compulsive tendencies, anxiety, depression and psychosis have been observed.[2][5] Some individuals may also experience motor and movement disorders.[2] Epilepsy and seizure disorders affect over one-third of individuals, with neuroimaging studies occasionally revealing non-specific findings.[4] Infants may experience feeding difficulties and constipation due to decreased muscle tone.[4]
Most individuals affected by Beck–Fahrner syndrome exhibit similar craniofacial anomalies, including myopathic facial changes, long face syndrome with a broad forehead, protruding ears and a high-arched palate. Musculoskeletal abnormalities encompass kyphosis, scoliosis, hyperflexible joints, hip misalignment and flat feet. Eye involvement can lead to vision, movement and alignment abnormalities,[4] while ear involvement may result in hearing loss.[1][3] Additional associated features comprise congenital heart defects, pyloric stenosis, inguinal hernia, hypospadias and undescended testis.[4] Overgrowth may manifest in some individuals, presenting with characteristics such a larger head size and tall stature; rarely this may be correlated with enlarged kidneys and heart. Conversely, undergrowth has also been reported, associated with a smaller head size and short stature.[2][5]
Genetics
The TET3 gene encodes the tet methylcytosine dioxygenase 3 (TET3) protein.[upper-alpha 1][6] TET3 facilitates conversion of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC),[7] which is an intermediate step in DNA demethylation.[8] TET3 is produced in embryonic stem cells during embryogenesis, where it contributes to the development of the nervous system by sustaining neural stem cells and promoting maturation of early neural cells.[9]
Beck–Fahrner syndrome is caused by specific mutations in the TET3 gene on chromosome 2 (2p13.1). These mutations can be homozygous (two identical mutated copies), heterozygous (one normal copy and one mutated copy), or compound heterozygous (two different mutated copies).[10] The mutations—which can be of various types like nonsense, missense or framshift—disrupt the normal DNA demethylation process during early embryonic development and the formation of the nervous system.[2] It can either occur spontaneously (de novo) due to new genetic mutations or be inherited in a way where one copy of the mutated gene is sufficient to cause the condition (autosomal dominant). The signs and symptoms may vary among individuals due to differences in gene expression and partial loss of gene function.[4]
Diagnosis
Beck–Fahrner syndrome shares clinical findings with several genetic disorders. These include Bainbridge–Ropers syndrome, Fragile X syndrome, Heyn–Sproule–Jackson syndrome, Kabuki syndrome, Luscan–Lumish syndrome, Malan syndrome, Sotos syndrome and Tatton-Brown–Rahman syndrome.[4] There is no consensus on diagnostic criteria specific to Beck–Fahrner syndrome.[4] Diagnosis involves confirming the presence of TET3 gene mutations in conjunction with the observation of typical clinical symptoms.[11]
Various molecular and genetic testing methods are employed to identify mutations or variants associated with Beck–Fahrner syndrome. These may include multigene panels incorporating the TET3 gene, whole genome sequencing, exome sequencing, sequence analysis, and single-gene testing followed by targeted gene deletion or duplication analysis. GeneReviews recommends exome sequencing as the diagnostic test of choice due to the recent delineation of the condition and the limited availability of TET3 gene analysis on most multigene panels.[4] Levy et al. (2021) identified a distinct DNA methylation profile or epigenetic signature (episignature)—demonstrating DNA hypermethylation—specific to Beck–Fahrner syndrome.[1] This episignature can be assessed through whole blood genome-wide DNA methylation analysis and may serve as a tool to confirm the pathogenicity of a TET3 variant of uncertain significance.[12][4]
Management
The management approach for Beck–Fahrner syndrome is primarily supportive, with a focus on improving the quality of life. The care is coordinated by medical genetics and pediatrics, involving a multidisciplinary team of specialists. Depending on specific symptoms, various medical specialists may be involved, including neurology for seizures, cardiology for heart defects and orthopedic surgery for musculoskeletal issues. Early interventions, such as autism therapies and participation in special education programs like behavior therapy and speech therapy, can help manage developmental and cognitive issues. Genetic counseling plays a role in educating patients and their families about the condition, assessing the risk of other family members having the disorder, offering guidance on family planning, and providing information on prenatal testing. Furthermore, social work assists patients and families in exploring palliative, respite and nursing home care options when necessary.[4]
History
Beck–Fahrner syndrome, initially termed "TET3 deficiency",[2] was first described in 2020.[13] It was the first human disorder of DNA demethylation to be delineated.[2] As of 2023, approximately 50 individuals have been diagnosed with this condition.[14]
Notes
- ↑ "TET" enzymes are a family of "ten-eleven translocation" methylcytosine dioxygenases that help in mediating active DNA demethylation.
References
- ↑ 1.0 1.1 1.2 Levy, Michael A. et al. (2021-11-08). "Deficiency of TET3 leads to a genome-wide DNA hypermethylation episignature in human whole blood". npj Genomic Medicine (Springer Nature) 6 (1): 92. doi:10.1038/s41525-021-00256-y. PMID 34750377.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Beck, David B. et al. (2020-01-09). "Delineation of a human mendelian disorder of the DNA demethylation machinery: TET3 deficiency". American Journal of Human Genetics (American Society of Human Genetics) 106 (2): 234–245. doi:10.1016/j.ajhg.2019.12.007. PMID 31928709.
- ↑ 3.0 3.1 Seyama, Rie et al. (2021-11-01). "Two families with TET3-related disorder showing neurodevelopmental delay with craniofacial dysmorphisms". Journal of Human Genetics (Japan Society of Human Genetics) 67 (3): 157–164. doi:10.1038/s10038-021-00986-y. PMID 34719681. https://www.nature.com/articles/s10038-021-00986-y. Retrieved 2023-07-16.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 Fahrner, Jill A. (2023-05-18). TET3-related Beck–Fahrner syndrome. National Center for Biotechnology Information. PMID 37200470. https://www.ncbi.nlm.nih.gov/books/NBK591837/. Retrieved 2023-07-15.
- ↑ 5.0 5.1 "Beck–Fahrner syndrome". National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/medgen/1711894.
- ↑ Wu, Xiaoji; Zhang, Yi (2017-09-01). "TET-mediated active DNA demethylation: mechanism, function and beyond". Nature Reviews Genetics (Springer Nature) 18 (9): 517–534. doi:10.1038/nrg.2017.33. PMID 28555658. https://www.nature.com/articles/nrg.2017.33. Retrieved 2023-07-30.
- ↑ Wang, Zhiqin et al. (2016-03-07). "DNA methylation dynamics in neurogenesis". Epigenomics (Future Medicine) 8 (3): 401–414. doi:10.2217/epi.15.119. PMID 26950681.
- ↑ MacArthur, Ian C.; Dawlaty, Meelad M. (2021-02-18). "TET enzymes and 5-hydroxymethylcytosine in neural progenitor cell biology and neurodevelopment". Frontiers in Cell and Developmental Biology (Frontiers Media) 9: 645335. doi:10.3389/fcell.2021.645335. PMID 33681230.
- ↑ Li, Ting et al. (2014-05-18). "Critical role of TET3 in neural progenitor cell maintenance and terminal differentiation". Molecular Neurobiology (Springer Nature) 51 (1): 142–154. doi:10.1007/s12035-014-8734-5. PMID 24838624. https://link.springer.com/article/10.1007/s12035-014-8734-5. Retrieved 2023-07-30.
- ↑ "Beck–Fahrner syndrome; BEFAHRS". Johns Hopkins University School of Medicine. 2020-03-04. https://www.omim.org/entry/618798.
- ↑ Richards, Sue et al. (2015-05-01). "Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology". Genetics in Medicine (American College of Medical Genetics and Genomics) 17 (5): 405–424. doi:10.1038/gim.2015.30. PMID 25741868.
- ↑ Sadikovic, Bekim et al. (2021-06-01). "Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders". Genetics in Medicine (American College of Medical Genetics) 23 (6): 1065–1074. doi:10.1038/s41436-020-01096-4. PMID 33547396.
- ↑ "What is Beck–Fahrner Syndrome? (BEFAHRS)". Beck–Fahrner Syndrome Foundation. 2021-10-10. https://beckfahrner.org/about/.
- ↑ Doherty, Megan (2023-06-16). "Ashley Clifford is one of only 50 people with Beck–Fahrner syndrome". The Canberra Times (Australian Community Media). https://www.canberratimes.com.au/story/8235956/canberra-girl-one-of-only-50-in-world-with-rare-syndrome/.
 |

