Chemistry:Ruthenium pentacarbonyl
| |
| Names | |
|---|---|
| Other names
Pentacarbonylruthenium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
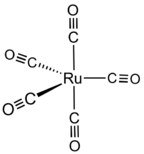
| Ru(CO)5 | |
| Molar mass | 241.12 |
| Appearance | colorless liquid |
| Melting point | −16–17[1] °C (3–63 °F; 257–290 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ruthenium pentacarbonyl is the organoruthenium compound with the formula Ru(CO)5. It is a colorless, light-sensitive liquid that readily decarbonylates upon standing at room temperature. It is of academic interest as an intermediate for the synthesis of metal carbonyl complexes.[2]
Preparation
Ru(CO)5 was originally prepared by carbonylation of ruthenium salts in the presence of a reducing agent.[3] A more recent preparation involves photolysis of triruthenium dodecacarbonyl in the presence of carbon monoxide:[2]
- Ru3(CO)12 + 3 CO ⇌ 3 Ru(CO)5
It is characterized by two intense νCO bands in the IR spectrum at 2038 and 2003 cm−1 (hexane solution).[2]
Comparisons of M(CO)5 (M = Fe, Ru, Os)
Whereas Fe(CO)5 is completely robust at room temperature, samples of Ru(CO)5 are typically reddish owing to contamination by Ru3(CO)12. The conversion is rapid in solution. Os(CO)5 requires heating to 80 °C to effect conversion to triosmium dodecacarbonyl.[1]
References
- ↑ 1.0 1.1 Rushman, Paul; Van Buuren, Gilbert N.; Shiralian, Mahmoud; Pomeroy, Roland K. (1983). "Properties of the Pentacarbonyls of Ruthenium and Osmium". Organometallics 2 (5): 693–694. doi:10.1021/om00077a026.
- ↑ 2.0 2.1 2.2 Adams, R. D.; Barnard, T. S.; Cortopassi, J. E.; Wu, W.; Li, Z. "Platinum-ruthenium carbonyl cluster complexes" Inorganic Syntheses 1998, volume 32, pp. 280-284. doi:10.1002/9780470132630.ch44
- ↑ W. Manchot, Wilhelm J. Manchot "Darstellung von Rutheniumcarbonylen und -nitrosylen" Zeitschrift für Anorganische und Allgemeine Chemie 1936, volume 226, pp. 385-415. doi:10.1002/zaac.19362260410
 |

