Chemistry:Tetramethyl orthosilicate
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetramethyl orthosilicate
| |||
| Other names
tetramethyl orthosilicate; methyl silicate; silicic acid, tetramethyl ester; silicon methoxide; TMOS
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| SiC4H12O4 | |||
| Molar mass | 152.25 | ||
| Appearance | colourless liquid | ||
| Density | 1.032 | ||
| Melting point | 4 to 5 °C (39 to 41 °F; 277 to 278 K) | ||
| Boiling point | 121 to 122 °C (250 to 252 °F; 394 to 395 K) | ||
| organic solvents | |||
| Vapor pressure | 12 mmHg (25°C)[1] | ||
| Hazards | |||
| Main hazards | toxic | ||
| Flash point | 96 °C; 205 °F; 369 K[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
TWA 1 ppm (6 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
| Related compounds | |||
Other cations
|
Trimethyl borate Trimethyl phosphite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
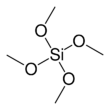
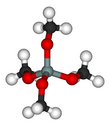
Tetramethyl orthosilicate (TMOS) is the chemical compound with the formula Si(OCH3)4. This molecule consists of four methoxy groups bonded to a silicon atom. The basic properties are similar to the more popular tetraethyl orthosilicate, which is usually preferred because the product of hydrolysis, ethanol, is less toxic than methanol.
Tetramethyl orthosilicate hydrolyzes to SiO2:
In organic synthesis, Si(OCH3)4 has been used to convert ketones and aldehydes to the corresponding ketals and acetals, respectively.[2]
Safety
The hydrolysis of Si(OCH3)4 produces insoluble SiO2 and CH3OH (methanol). Even at low concentrations inhalation causes lung lesions, and at slightly higher concentrations eye contact with the vapor causes blindness[citation needed]. Worse, at low concentrations (200 ppm/15 min) the damage is often insidious, with onset of symptoms hours after exposure.[3] The mode of action is the precipitation of silica in the eyes and/or lungs[citation needed]. Contrary to common information, including several erroneous MSDS sheets, the methanol produced is only a risk through chronic exposure and is a comparatively small concern. The mechanisms of methanol toxicity are well established, methanol causes blindness via conversion to formaldehyde, then to toxic formic acid in the liver; methanol splashes to the eye cause only moderate and reversible eye irritation.[4]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 NIOSH Pocket Guide to Chemical Hazards. "#0428". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0428.html.
- ↑ Sakurai, H. "Silicon(IV) Methoxide" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons. doi:10.1002/047084289X.rs012
- ↑ "Tetramethyl silicate". National Library of Medicine - Hazardous Substances Data Bank. National Library of Medicine. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~v3cqfN:1:FULL. Retrieved 11 February 2013.
- ↑ "Methanol". Hazardous Substances Data Bank - National Library of Medicine. National Library of Medicine. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~Bjt41r:1. Retrieved 11 February 2013.
External links
 |