Chemistry:Hydroxyacetone
From HandWiki
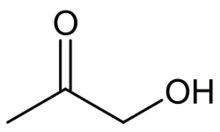
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Hydroxypropan-2-one | |
| Other names
1-Hydroxy-2-propanone
Acetol | |
| Identifiers | |
3D model (JSmol)
|
|
| 605368 | |
| ChemSpider | |
| EC Number |
|
| |
| |
| Properties | |
| C3H6O2 | |
| Molar mass | 74.08 |
| Appearance | Colourless liquid |
| Odor | sweet |
| Density | 1.059 g/cm3[1] |
| Melting point | −17 °C (1 °F; 256 K) |
| Boiling point | 145–146 °C (293–295 °F; 418–419 K) |
| Vapor pressure | 7.5 hPa at 20 °C[2] |
Refractive index (nD)
|
1.415[1] |
| Hazards | |
| H226[2] | |
| Flash point | 56 °C (closed cup)[2] |
| Explosive limits | Upper limit: 14.9%(V) Lower limit: 3%(V)[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2200 mg/kg (rat, oral)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxyacetone, also known as acetol, is an organic chemical consisting of a primary alcohol substituent on acetone. It is an α-hydroxyketone, also called a ketol, and is the simplest hydroxy ketone structure.
Hydryoxyacetone can be produced by degradation of various sugars. In foods, it is formed by the Maillard reaction, and can react further to form other compounds with various aromas.[4] As such it finds some use as a flavoring.
Hydroxyacetone is commercially available but may be also be synthesized on a laboratory scale by a substitution reaction on bromoacetone.[5] It undergoes rapid polymerization, including forming a hemiacetal cyclic dimer. Under alkaline conditions, it undergoes a rapid aldol condensation.
See also
- Acyloin, the simplest secondary α-hydroxy ketone.
References
- ↑ 1.0 1.1 Nodzu, Ryuzaburo (1935). "On the Action of Phosphate Upon Hexoses. I. The Formation of Acetol From Glucose in Acidic Solution of Potassium Phosphate". Bul. Chem. Soc. Jpn. 10 (3): 122–130. doi:10.1246/bcsj.10.122.
- ↑ 2.0 2.1 2.2 2.3 Sigma-Aldrich Co., Hydroxyacetone. Retrieved on 2 July 2015.
- ↑ Smyth, H. F., Jr; Carpenter, C. P. (January 1948). "Further experience with the range finding test in the industrial toxicology laboratory". The Journal of industrial hygiene and toxicology 30 (1): 63–8. PMID 18895731.
- ↑ Nursten, Harry E. (1998). "The Mechanism of Formation of 3-Methylcyclopent-2-en-2-olone". in O'Brien, J.; Nursten, H. E.; Crabbe, M. J. et al.. The Maillard Reaction in Foods and Medicine. Elsevier. pp. 65–68. ISBN 9781845698447.
- ↑ Levene, P. A.; Walti, A. (1930). "Acetol". Organic Syntheses 10: 1. http://www.orgsyn.org/demo.aspx?prep=cv2p0005.; Collective Volume, 2, pp. 5

