Medicine:Hay–Wells syndrome
| Hay–Wells syndrome | |
|---|---|
| Other names | Ankyloblepharon-ectodermal defects-cleft lip/palate syndrome |
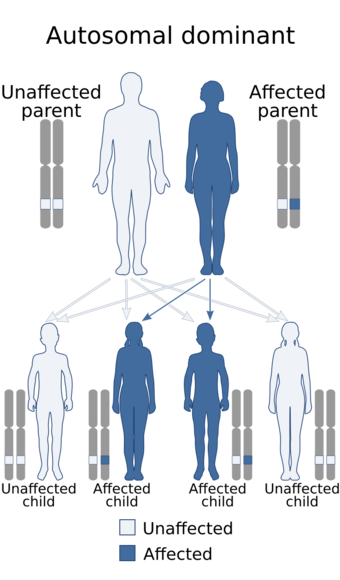 | |
| Hay–Wells syndrome has an autosomal dominant pattern of inheritance | |
Hay–Wells syndrome (also known as AEC syndrome; see Naming) is one of at least 150 known types of ectodermal dysplasia.[1][2] These disorders affect tissues that arise from the ectodermal germ layer, such as skin, hair, and nails.
Genetics
Hay–Wells syndrome is autosomal dominant,[3] caused by a missense mutation in the Sterile alpha motif (SAM) of the TP73L (p63) gene which encodes for a protein-protein interaction domain.[3] It is a very rare disorder.
Hay–Wells syndrome is an autosomal dominant pattern of inheritance.[4] The syndrome is thought to arise from a missense mutation in a gene pivotal for the proper development of craniofacial structures and extremities, as well as skin differentiation.[5] Specifically, mutations within the Tumor Protein 63 gene have been implicated in Hay–Wells syndrome.[6]
Residing on the long-arm of chromosome 3, the Tumor Protein 63 (TP63) gene is critical for proper development and homeostasis of stratified epithelia.[7] In Hay–Wells syndrome, and other ectodermal dysplasia disorders, a missense, nonsense, or insertion mutation has occurred in the TP63 gene. Currently, no deletion or duplication mutations have been detected in such disorders.[6] Although ectodermal dysplasia disorders result from heterozygous mutations in TP63, compromised epidermal differentiation with epidermal decay is representative of Hay-Wells patients but is hardly observed in other syndromes. In contrast, severe abnormalities characteristic of other ectodermal dysplasia disorders (i.e. limb abnormalities in EEC) are not seen in Hay-Wells patients.[8][9][10]
Proteomics
TP63 encodes for the p63 transcription factor, which is implicated in proliferation, differentiation, apoptosis, regular cell maintenance, and cell adhesion. Specifically, p63 is expressed within early keratinocytes and the embryonic ectodermal ridge during development. Thus, p63 is believed to play a pivotal role in the development and maintenance of the epidermis.[11] Reported mutations that have resulted in Hay–Wells syndrome have occurred within the sterile alpha motif (SAM) and the transactivation inhibitory (TI) domains of the p63-coding region.[citation needed] The SAM domain of p63 is thought to be imperative for protein-protein interactions, while the TI domain may play a role in the repression of other isoforms of p63.[12][13] Recent work has shown that mutations within these domains lead to repression of other known transcriptional activators of epidermal differentiation. These transcription activators include: GRHL3, HOPX, PRDM1, KLF4, and ZNF750.[10][14][15] Most notably, Hay-Wells-type p63 mutations cause irregular repression of the genes that encode for ZNF750. The down-regulation of ZNF750 has been shown to hinder the expression of the other before mentioned differentiation-activators such as HOPX, PRDM1, KLF4, and GRHL3. In contrast, recapitulating the expression of ZNF750 leads to significant rescue of normal epidermal differentiation.[10]
Phenotype
Hay–Wells syndrome is the result of the invariant mutations of the p63 transcription factor that have been previously identified. Due to the diminished activities of p63, patients can experience a host of symptoms related to the operation of keratinocytes. In particular, the hypopigmentation observed in several Hay-Wells patients is believed to be the result of improperly developed keratinocytes not being able to properly interact with melanocytes.[16] However, as it stands, this display of Hay–Wells syndrome has not been entirely comprehended. Most noted are the abnormal development of hair, teeth, glands, and nails.[4]
Diagnosis
In HWS, the hair is coarse and sparse, eyelashes are sparse or absent, nails may be absent or malformed, and teeth may be small and malformed. There may be fewer than normal sweat glands and they may produce little sweat, a condition known generally as hypohidrosis. Chronic inflammatory dermatitis of the scalp is a common symptom.[17]
Two features differentiate HWS from other ectodermal displasias. First, the syndrome is associated with cleft palate, and, less often, cleft lip. Second, the edges of the upper and lower eyelid grow bands of fibrous tissue, often causing them to be fused together. This condition in the eyelids is called ankyloblepharon filiforme adnatum.[citation needed]
Management
Etymology
Hay–Wells syndrome is also known as AEC syndrome; this is short for "ankyloblepharon–ectodermal dysplasia–clefting syndrome", "ankyloblepharon filiforme adnatum–ectodermal dysplasia–cleft palate syndrome",[18] "ankyloblepharon–ectodermal defects–cleft lip/palate (AEC) syndrome",[9] "ankyloblepharon–ectodermal defect–cleft lip and/or palate syndrome",[19] or "ankyloblepharon ectodermal dysplasia and clefting".[20] Hay–Wells syndrome, or Ankyloblepharon-Ectodermal Dysplasia-Clefting (AEC) syndrome, is one of the least known form of ectodermal dysplasia; a collection of inherited diseases that cause atypical development of nails, glands, teeth, and hair. Fewer than 100 affected individuals have been described in the medical literature. Males and females are equally affected by Hay–Wells syndrome. No demographic has been shown to be especially susceptible to the syndrome.[21] Symptoms are apparent at birth, or become apparent when atypical development of teeth occurs.[4] Major symptoms of Hay–Wells syndrome include: sparse hair and eyelashes, missing teeth, cleft palate, cleft lip with fusing of the upper and lower eyelids, and deformed nails.[5][22] Therefore, a diagnosis of Hay–Wells syndrome is largely based upon the physical clinical presentation of the patient.[22]
See also
- TP73L
- List of cutaneous conditions
- List of dental abnormalities associated with cutaneous conditions
References
- ↑ Online Mendelian Inheritance in Man (OMIM) 106260
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology (10th ed.). Saunders. p. 571. ISBN 978-0-7216-2921-6.
- ↑ 3.0 3.1 McGrath, John A.; Duijf, Pascal H.G.; Doetsch, Volker; Irvine, Alan D.; de Waal, Rob; Vanmolkot, Kaate R.J.; Wessagowit, Vesarat; Kelly, Alexander et al. (2001). "Hay–Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63". Human Molecular Genetics 10 (3): 221–9. doi:10.1093/hmg/10.3.221. PMID 11159940.
- ↑ 4.0 4.1 4.2 Nagaveni, NB; Umashankara, KV (2011). "Hay-Wells syndrome of ectodermal dysplasia: A rare autosomal dominant disorder". Indian Journal of Human Genetics 17 (3): 245–6. doi:10.4103/0971-6866.92084. PMID 22346004.
- ↑ 5.0 5.1 Macias, Emilio; de Carlos, Felix; Cobo, Juan (2006). "Hay–Wells syndrome (AEC): a case report". Oral Diseases 12 (5): 506–8. doi:10.1111/j.1601-0825.2006.01227.x. PMID 16910923.
- ↑ 6.0 6.1 Rinne, Tuula; Bolat, Emine; Meijer, Rowdy; Scheffer, Hans; van Bokhoven, Hans (2009). "Spectrum ofp63mutations in a selected patient cohort affected with ankyloblepharon-ectodermal defects-cleft lip/palate syndrome (AEC)". American Journal of Medical Genetics Part A 149A (9): 1948–51. doi:10.1002/ajmg.a.32793. PMID 19676060.
- ↑ Senoo, Makoto; Pinto, Filipa; Crum, Christopher P.; McKeon, Frank (2007). "p63 Is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia". Cell 129 (3): 523–36. doi:10.1016/j.cell.2007.02.045. PMID 17482546.
- ↑ Brunner, H G; Hamel, B C J; van Bokhoven, H (2002). "The p63 gene in EEC and other syndromes". Journal of Medical Genetics 39 (6): 377–81. doi:10.1136/jmg.39.6.377. PMID 12070241.
- ↑ 9.0 9.1 Julapalli, Meena R.; Scher, Richard K.; Sybert, Virginia P.; Siegfried, Elaine C.; Bree, Alanna F. (2009). "Dermatologic findings of ankyloblepharon-ectodermal defects-cleft lip/palate (AEC) syndrome". American Journal of Medical Genetics Part A 149A (9): 1900–6. doi:10.1002/ajmg.a.32797. PMID 19681128.
- ↑ 10.0 10.1 10.2 Zarnegar, Brian J.; Webster, Dan E.; Lopez-Pajares, Vanessa; Vander Stoep Hunt, Brook; Qu, Kun; Yan, Karen J.; Berk, David R.; Sen, George L. et al. (2012). "Genomic Profiling of a Human Organotypic Model of AEC Syndrome Reveals ZNF750 as an Essential Downstream Target of Mutant TP63". The American Journal of Human Genetics 91 (3): 435–43. doi:10.1016/j.ajhg.2012.07.007. PMID 22922031.
- ↑ Chan, I.; McGrath, J. A.; Kivirikko, S. (2005). "Rapp–Hodgkin syndrome and the tail of p63". Clinical and Experimental Dermatology 30 (2): 183–6. doi:10.1111/j.1365-2230.2004.01715.x. PMID 15725251.
- ↑ Koster, Maranke I; Roop, Dennis R. (2004). "The role of p63 in development and differentiation of the epidermis". Journal of Dermatological Science 34 (1): 3–9. doi:10.1016/j.jdermsci.2003.10.003. PMID 14757276.
- ↑ van Bokhoven, Hans; Brunner, Han G. (2002). "Splitting p63". The American Journal of Human Genetics 71 (1): 1–13. doi:10.1086/341450. PMID 12037717.
- ↑ Birnbaum, Ramon Y; Zvulunov, Alex; Hallel-Halevy, Dafna; Cagnano, Emanuella; Finer, Gal; Ofir, Rivka; Geiger, Dan; Silberstein, Eldad et al. (2006). "Seborrhea-like dermatitis with psoriasiform elements caused by a mutation in ZNF750, encoding a putative C2H2 zinc finger protein". Nature Genetics 38 (7): 749–51. doi:10.1038/ng1813. PMID 16751772.
- ↑ Yang, Chi-Fan; Hwu, Wuh-Liang; Yang, Li-Cheng; Chung, Wen-Hung; Chien, Yin-Hsiu; Hung, Chia-Fu; Chen, Hung-Chih; Tsai, Pei-Joung et al. (2008). "A Promoter Sequence Variant of ZNF750 Is Linked with Familial Psoriasis". Journal of Investigative Dermatology 128 (7): 1662–8. doi:10.1038/jid.2008.1. PMID 18256691.
- ↑ Seiberg, M.; Paine, C.; Sharlow, E.; Andrade-Gordon, P.; Costanzo, M.; Eisinger, M.; Shapiro, S.S. (2000). "The Protease-Activated Receptor 2 Regulates Pigmentation via Keratinocyte-Melanocyte Interactions". Experimental Cell Research 254 (1): 25–32. doi:10.1006/excr.1999.4692. PMID 10623462.
- ↑ Ectodermal Dysplasia~clinical at eMedicine
- ↑ Freedberg (2003). Fitzpatrick's Dermatology in General Medicine (6th ed.). McGraw-Hill. p. 518. ISBN 978-0-07-138076-8.
- ↑ Motil, Kathleen J.; Fete, Timothy J. (2009). "Growth, nutritional, and gastrointestinal aspects of ankyloblepharon-ectodermal defect-cleft lip and/or palate (AEC) syndrome". American Journal of Medical Genetics Part A 149A (9): 1922–5. doi:10.1002/ajmg.a.32789. PMID 19676058.
- ↑ Koster, Maranke I.; Marinari, Barbara; Payne, Aimee S.; Kantaputra, Piranit N.; Costanzo, Antonio; Roop, Dennis R. (2009). "ΔNp63 knockdown mice: A mouse model for AEC syndrome". American Journal of Medical Genetics Part A 149A (9): 1942–7. doi:10.1002/ajmg.a.32794. PMID 19681108.
- ↑ Bissonnette, Bruno; Luginbuehl, Igor; Marciniak, Bruno; Dalens, Bernard J. (2006), "AEC Syndrome", Syndromes: Rapid Recognition and Perioperative Implications (New York, NY: The McGraw-Hill Companies), http://accessanesthesiology.mhmedical.com/content.aspx?aid=58061871, retrieved 2021-02-23
- ↑ 22.0 22.1 Hay, R.J.; Wells, R.S. (1976). "The syndrome of ankyloblepharon, ectodermal defects and cleft lip and palate: an autosomal dominant condition". British Journal of Dermatology 94 (3): 277–89. doi:10.1111/j.1365-2133.1976.tb04384.x. PMID 946410.
Further reading
- Clements, S.E.; Techanukul, T.; Holden, S.T.; Mellerio, J.E.; Dorkins, H.; Escande, F.; McGrath, J.A. (2010). "Rapp–Hodgkin and Hay–Wells ectodermal dysplasia syndromes represent a variable spectrum of the same genetic disorder". British Journal of Dermatology 163 (3): 624–9. doi:10.1111/j.1365-2133.2010.09859.x. PMID 20491771.
- Korf, B.R. (2011). "Principles of Genetics". in Goldman, L; Ausiello, D. Cecil Medicine (24th ed.). Philadelphia: Saunders Elsevier.
- Sathyamurthy, Aruna; Freund, Stefan M. V.; Johnson, Christopher M.; Allen, Mark D.; Bycroft, Mark (2011). "Structural basis of p63α SAM domain mutants involved in AEC syndrome". FEBS Journal 278 (15): 2680–8. doi:10.1111/j.1742-4658.2011.08194.x. PMID 21615690.
- GeneReviews/NCBI/NIH/UW entry on Ankyloblepharon-Ectodermal Defects-Cleft Lip/Palate Syndrome or AEC Syndrome, Hay-Wells Syndrome. Includes: Rapp–Hodgkin Syndrome
External links
| Classification | |
|---|---|
| External resources |
 |

