Medicine:Mild androgen insensitivity syndrome
This article may contain an excessive number of citations. (September 2021) (Learn how and when to remove this template message) |
| Mild androgen insensitivity syndrome | |
|---|---|
| Other names | Undervirilized male syndrome |
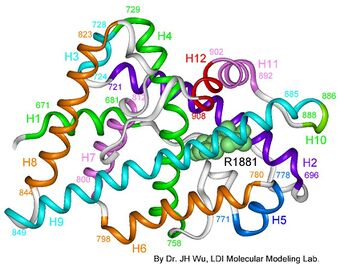 | |
| AIS results when the function of the androgen receptor (AR) is impaired. The AR protein (pictured) mediates the effects of androgens in the human body. | |
Mild androgen insensitivity syndrome (MAIS) is a condition that results in a mild impairment of the cell's ability to respond to androgens.[1][2][3] The degree of impairment is sufficient to impair spermatogenesis and / or the development of secondary sexual characteristics at puberty in males, but does not affect genital differentiation or development. Female genital and sexual development is not significantly affected by the insensitivity to androgens;[3][4] as such, MAIS is only diagnosed in males.[1] The clinical phenotype associated with MAIS is a normal male habitus with mild spermatogenic defect and / or reduced secondary terminal hair.[1][5][6][7][8][9]
MAIS is one of three types of androgen insensitivity syndrome, which is divided into three categories that are differentiated by the degree of genital masculinization: complete androgen insensitivity syndrome (CAIS) is indicated when the external genitalia is phenotypically female, mild androgen insensitivity syndrome (MAIS) is indicated when the external genitalia is phenotypically male, and partial androgen insensitivity syndrome (PAIS) is indicated when the external genitalia is partially, but not fully masculinized.[1][2][5][6][7][10][11][12][13]
Androgen insensitivity syndrome is the largest single entity that leads to 46,XY undermasculinization.[14]
Signs and symptoms
Individuals with mild (or minimal) androgen insensitivity syndrome (grade 1 on the Quigley scale) are born phenotypically male, with fully masculinized genitalia; this category of androgen insensitivity is diagnosed when the degree of androgen insensitivity in an individual with a 46,XY karyotype is great enough to impair virilization or spermatogenesis, but is not great enough to impair normal male genital development.[1][5][6][9] MAIS is the mildest and least known form of androgen insensitivity syndrome.[5][16]
The existence of a variant of androgen insensitivity that solely affected spermatogenesis was theoretical at first.[17] Cases of phenotypically normal males with isolated spermatogenic defect due to AR mutation were first detected as the result of male infertility evaluations.[1][13][18][19] Until then, early evidence in support of the existence of MAIS was limited to cases involving a mild defect in virilization,[15][20] although some of these early cases made allowances for some degree of impairment of genital masculinization, such as hypospadias or micropenis.[21][22][23] It is estimated that 2-3% of infertile men have AR gene mutations.[6] It is also estimated that MAIS is responsible for 40% of male infertility.[24]
Examples of MAIS phenotypes include isolated infertility (oligospermia or azoospermia),[5][7] mild gynecomastia in young adulthood, decreased secondary terminal hair, high pitched voice, or minor hypospadias repair in childhood.[1][25] The external male genitalia (penis and scrotum) are otherwise normal in individuals with MAIS.[1][5][6][9] Internal genitalia, including Wolffian structures (the epididymides, vasa deferentia, and seminal vesicles) and the prostate, is also normal, although the bitesticular volume of infertile men (both with and without MAIS) is diminished;[6] male infertility is associated with reduced bitesticular volume, varicocele, retractile testes, low ejaculate volume, male accessory gland infections (MAGI), and mumps orchitis.[6] The incidence of these features in infertile men with MAIS is similar to that of infertile men without MAIS.[6] MAIS is not associated with Müllerian remnants.
Spinal and bulbar muscular atrophy
Spinal and bulbar muscular atrophy (SBMA), also known as Kennedy's disease, is a severe neurodegenerative syndrome that is associated with a particular mutation of the androgen receptor's polyglutamine tract called a trinucleotide repeat expansion.[26][27] SBMA results when the length of the polyglutamine tract exceeds 40 repetitions.[28]
Although technically a variant of MAIS, SBMA's presentation is not typical of androgen insensitivity; symptoms do not occur until adulthood and include neuromuscular defects as well as signs of androgen inaction.[26] Neuromuscular symptoms include progressive proximal muscle weakness, atrophy, and fasciculations. Symptoms of androgen insensitivity experienced by men with SBMA are also progressive [26] and include testicular atrophy, severe oligospermia or azoospermia, gynecomastia, and feminized skin changes [29] despite elevated androgen levels.[1] Disease onset, which usually affects the proximal musculature first, occurs in the third to fifth decades of life, and is often preceded by muscular cramps on exertion, tremor of the hands, and elevated muscle creatine kinase.[30] SBMA is often misdiagnosed as amyotrophic lateral sclerosis (ALS) (also known as Lou Gehrig's disease).[27]
The symptoms of SBMA are thought to be brought about by two simultaneous pathways involving the toxic misfolding of proteins and loss of AR functionality.[1] The polyglutamine tract in affected pedigrees tends to increase in length over generations, a phenomenon known as "anticipation",[31] leading to an increase in the severity of the disease as well as a decrease in the age of onset for each subsequent generation of a family affected by SBMA.[26]
Comorbidity
All forms of androgen insensitivity are associated with infertility, though exceptions have been reported for both the mild and partial forms.[4][5][7][33][34][35] Lifespan is not thought to be affected by AIS.[1]
Trinucleotide satellite lengths and AR transcriptional activity
The androgen receptor gene contains two polymorphic trinucleotide microsatellites in exon 1.[2] The first microsatellite (nearest the 5' end) contains 8 [36] to 60 [27][30] repetitions of the glutamine codon "CAG" and is thus known as the polyglutamine tract.[3] The second microsatellite contains 4 [37] to 31 [38] repetitions of the glycine codon "GGC" and is known as the polyglycine tract.[39] The average number of repetitions varies by ethnicity, with Caucasians exhibiting an average of 21 CAG repeats, and Blacks 18.[40] Disease states are associated with extremes in polyglutamine tract length; prostate cancer,[26] hepatocellular carcinoma,[41] and intellectual disabilities[36] are associated with too few repetitions, while spinal and bulbar muscular atrophy (SBMA) is associated with a CAG repetition length of 40 or more.[28] Some studies indicate that the length of the polyglutamine tract is inversely correlated with transcriptional activity in the AR protein, and that longer polyglutamine tracts may be associated with infertility[42][43][44] and undermasculinized genitalia.[45] However, other studies have indicated that no such correlation exists.[46][47][48][49][50][51] A comprehensive meta-analysis of the subject published in 2007 supports the existence of the correlation, and concluded that these discrepancies could be resolved when sample size and study design are taken into account.[11] Longer polyglycine tract lengths have also been associated with genital masculinization defects in some,[52][53] but not all,[54] studies.
Diagnosis
MAIS is only diagnosed in normal phenotypic males, and is not typically investigated except in cases of male infertility.[18] MAIS has a mild presentation that often goes unnoticed and untreated;[15] even with semenological, clinical and laboratory data, it can be difficult to distinguish between men with and without MAIS, and thus a diagnosis of MAIS is not usually made without confirmation of an AR gene mutation.[5] The androgen sensitivity index (ASI), defined as the product of luteinizing hormone (LH) and testosterone (T), is frequently raised in individuals with all forms of AIS, including MAIS, although many individuals with MAIS have an ASI in the normal range.[5] Testosterone levels may be elevated despite normal levels of luteinizing hormone.[15][20][25] Conversion of testosterone (T) to dihydrotestosterone (DHT) may be impaired, although to a lesser extent than is seen in 5α-reductase deficiency.[3] A high ASI in a normal phenotypic male,[46] especially when combined with azoospermia or oligospermia,[5][7] decreased secondary terminal hair,[27] and/or impaired conversion of T to DHT,[3] can be indicative of MAIS, and may warrant genetic testing.
Management
Due to its mild presentation, MAIS often goes unnoticed and untreated.[15] Management of MAIS is currently limited to symptomatic management; methods to correct a malfunctioning androgen receptor protein that result from an AR gene mutation are not currently available. Treatment includes surgical correction of mild gynecomastia, minor hypospadias repair, and testosterone supplementation.[1][15][55] Supraphysiological doses of testosterone have been shown to correct diminished secondary sexual characteristics in men with MAIS,[15] as well as to reverse infertility due to low sperm count.[55][56] As is the case with PAIS, men with MAIS will experience side effects from androgen therapy (such as the suppression of the hypothalamic-pituitary-gonadal axis) at a higher dosage than unaffected men. Careful monitoring is required to ensure the safety and efficacy of treatment.[15][57][58] Regular breast[57] and prostate[59] examinations may be necessary due to comorbid association with breast and prostate cancers.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Androgen resistance". Best Pract. Res. Clin. Endocrinol. Metab. 20 (4): 577–98. December 2006. doi:10.1016/j.beem.2006.11.003. PMID 17161333.
- ↑ 2.0 2.1 2.2 "Androgen insensitivity syndrome: clinical features and molecular defects". Hormones (Athens) 7 (3): 217–29. 2008. doi:10.14310/horm.2002.1201. PMID 18694860.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Androgen receptor defects: historical, clinical, and molecular perspectives". Endocr. Rev. 16 (3): 271–321. June 1995. doi:10.1210/edrv-16-3-271. PMID 7671849.
- ↑ 4.0 4.1 "An androgen receptor gene mutation (E653K) in a family with congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency as well as in partial androgen insensitivity". J. Clin. Endocrinol. Metab. 87 (6): 2623–8. June 2002. doi:10.1210/jcem.87.6.8518. PMID 12050225.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 "Detailed functional studies on androgen receptor mild mutations demonstrate their association with male infertility". Clin. Endocrinol. 68 (4): 580–8. April 2008. doi:10.1111/j.1365-2265.2007.03069.x. PMID 17970778.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 "Male infertility and androgen receptor gene mutations: clinical features and identification of seven novel mutations". Clin. Endocrinol. 65 (5): 606–10. November 2006. doi:10.1111/j.1365-2265.2006.02635.x. PMID 17054461.
- ↑ 7.0 7.1 7.2 7.3 7.4 "Male infertility and the involvement of the X chromosome". Hum. Reprod. Update 15 (6): 623–37. 2009. doi:10.1093/humupd/dmp023. PMID 19515807.
- ↑ "A novel mutation (N233K) in the transactivating domain and the N756S mutation in the ligand binding domain of the androgen receptor gene are associated with male infertility". Clin. Endocrinol. 54 (6): 827–34. June 2001. doi:10.1046/j.1365-2265.2001.01308.x. PMID 11422119.
- ↑ 9.0 9.1 9.2 "A novel sequence variation in the transactivation regulating domain of the androgen receptor in two infertile Finnish men". Fertil. Steril. 79 (Suppl 3): 1647–8. June 2003. doi:10.1016/s0015-0282(03)00256-5. PMID 12801573.
- ↑ "Incidental detection of Sertoli-Leydig cell tumor by FDG PET/CT imaging in a patient with androgen insensitivity syndrome". Ann Nucl Med 24 (1): 35–9. January 2010. doi:10.1007/s12149-009-0321-x. PMID 19957213.
- ↑ 11.0 11.1 "Male infertility and variation in CAG repeat length in the androgen receptor gene: a meta-analysis". J. Clin. Endocrinol. Metab. 92 (11): 4319–26. November 2007. doi:10.1210/jc.2007-1110. PMID 17684052.
- ↑ "Impaired nuclear translocation, nuclear matrix targeting, and intranuclear mobility of mutant androgen receptors carrying amino acid substitutions in the deoxyribonucleic acid-binding domain derived from androgen insensitivity syndrome patients". J. Clin. Endocrinol. Metab. 90 (11): 6162–9. November 2005. doi:10.1210/jc.2005-0179. PMID 16118342.
- ↑ 13.0 13.1 "Molecular pathology of the androgen receptor in male (in)fertility". Reprod. Biomed. Online 10 (1): 42–8. January 2005. doi:10.1016/S1472-6483(10)60802-4. PMID 15705293.
- ↑ "Assessment of the gonadotrophin-gonadal axis in androgen insensitivity syndrome". Arch. Dis. Child. 80 (4): 324–9. April 1999. doi:10.1136/adc.80.4.324. PMID 10086936.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 "Impaired spermatogenesis is not an obligate expression of receptor-defective androgen resistance". Am. J. Med. Genet. 32 (1): 100–4. January 1989. doi:10.1002/ajmg.1320320121. PMID 2705470.
- ↑ "Analysis of the transactivation domain of the androgen receptor in patients with male infertility". Clin. Genet. 54 (3): 185–92. September 1998. doi:10.1111/j.1399-0004.1998.tb04282.x. PMID 9788719.
- ↑ "Azoospermia associated with a mutation in the ligand-binding domain of an androgen receptor displaying normal ligand binding, but defective trans-activation". J. Clin. Endocrinol. Metab. 83 (12): 4303–9. December 1998. doi:10.1210/jcem.83.12.5358. PMID 9851768.
- ↑ 18.0 18.1 "Complete androgen insensitivity syndrome--a review". J Pediatr Adolesc Gynecol 21 (6): 305–10. December 2008. doi:10.1016/j.jpag.2007.09.006. PMID 19064222.
- ↑ "Androgen receptor gene and male infertility". Hum. Reprod. Update 9 (1): 1–7. 2003. doi:10.1093/humupd/dmg003. PMID 12638777.
- ↑ 20.0 20.1 "A mutation of the androgen receptor associated with partial androgen resistance, familial gynecomastia, and fertility". J. Clin. Endocrinol. Metab. 66 (4): 754–61. April 1988. doi:10.1210/jcem-66-4-754. PMID 3346354.
- ↑ "Discordant measures of androgen-binding kinetics in two mutant androgen receptors causing mild or partial androgen insensitivity, respectively". J. Clin. Endocrinol. Metab. 84 (2): 805–10. February 1999. doi:10.1210/jcem.84.2.5453. PMID 10022458.
- ↑ "Human minimal androgen insensitivity with normal dihydrotestosterone-binding capacity in cultured genital skin fibroblasts: evidence for an androgen-selective qualitative abnormality of the receptor". Am. J. Hum. Genet. 36 (5): 965–78. September 1984. PMID 6333813.
- ↑ "An androgen receptor mutation causing androgen resistance in undervirilized male syndrome". J. Clin. Endocrinol. Metab. 79 (4): 1202–7. October 1994. doi:10.1210/jcem.79.4.7962294. PMID 7962294.
- ↑ "Androgen Insensitivity Syndrome | Encyclopedia.com". https://www.encyclopedia.com/medicine/diseases-and-conditions/pathology/androgen-insensitivity-syndrome.
- ↑ 25.0 25.1 "Phenotypic heterogeneity associated with identical mutations in residue 870 of the androgen receptor". Horm. Res. 57 (3–4): 90–3. 2002. doi:10.1159/000057958. PMID 12006704.
- ↑ 26.0 26.1 26.2 26.3 26.4 "Significance of the polyglutamine tract polymorphism in the androgen receptor". Urology 58 (5): 651–6. November 2001. doi:10.1016/S0090-4295(01)01401-7. PMID 11711330.
- ↑ 27.0 27.1 27.2 27.3 "A comprehensive endocrine description of Kennedy's disease revealing androgen insensitivity linked to CAG repeat length". J. Clin. Endocrinol. Metab. 87 (8): 3893–901. August 2002. doi:10.1210/jcem.87.8.8780. PMID 12161529.
- ↑ 28.0 28.1 "Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy". Nature 352 (6330): 77–9. July 1991. doi:10.1038/352077a0. PMID 2062380. Bibcode: 1991Natur.352...77S.
- ↑ "A family with adult spinal and bulbar muscular atrophy, X-linked inheritance and associated testicular failure". J. Neurol. Sci. 59 (3): 371–82. June 1983. doi:10.1016/0022-510X(83)90022-9. PMID 6683750.
- ↑ 30.0 30.1 "Trinucleotide repeats in the human androgen receptor: a molecular basis for disease". J. Mol. Endocrinol. 21 (3): 235–57. December 1998. doi:10.1677/jme.0.0210235. PMID 9845666.
- ↑ "Moderate instability of the trinucleotide repeat in spino bulbar muscular atrophy". Hum. Mol. Genet. 1 (4): 255–8. July 1992. doi:10.1093/hmg/1.4.255. PMID 1303195.
- ↑ "Case of sisters with complete androgen insensitivity syndrome and discordant Müllerian remnants". Fertil. Steril. 91 (3): 932.e15–8. March 2009. doi:10.1016/j.fertnstert.2008.09.027. PMID 18930210.
- ↑ "Male fertility is compatible with an Arg(840)Cys substitution in the AR in a large Chinese family affected with divergent phenotypes of AR insensitivity syndrome". J. Clin. Endocrinol. Metab. 87 (1): 347–51. January 2002. doi:10.1210/jcem.87.1.8167. PMID 11788673.
- ↑ "Complete androgen insensitivity syndrome with persistent Mullerian derivatives: a case report". J Obstet Gynaecol 25 (4): 403–5. May 2005. doi:10.1080/01443610500143226. PMID 16091340.
- ↑ "Preserved male fertility despite decreased androgen sensitivity caused by a mutation in the ligand-binding domain of the androgen receptor gene". J. Clin. Endocrinol. Metab. 85 (6): 2253–9. June 2000. doi:10.1210/jcem.85.6.6626. PMID 10852459.
- ↑ 36.0 36.1 "CAG repeat contraction in the androgen receptor gene in three brothers with mental retardation". Am. J. Med. Genet. 85 (3): 209–13. July 1999. doi:10.1002/(SICI)1096-8628(19990730)85:3<209::AID-AJMG4>3.0.CO;2-2. PMID 10398229.
- ↑ "Novel (60%) and recurrent (40%) androgen receptor gene mutations in a series of 59 patients with a 46,XY disorder of sex development". J. Clin. Endocrinol. Metab. 95 (4): 1876–88. April 2010. doi:10.1210/jc.2009-2146. PMID 20150575.
- ↑ "Codon-usage variants in the polymorphic (GGN)n trinucleotide repeat of the human androgen receptor gene". Hum. Genet. 101 (1): 43–6. November 1997. doi:10.1007/s004390050583. PMID 9385367.
- ↑ "Androgen insensitivity". Am. J. Med. Genet. 89 (4): 210–7. December 1999. doi:10.1002/(SICI)1096-8628(19991229)89:4<210::AID-AJMG5>3.0.CO;2-P. PMID 10727996.
- ↑ "Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups". Genomics 12 (2): 241–53. February 1992. doi:10.1016/0888-7543(92)90371-X. PMID 1740333.
- ↑ "Somatic mutations at the trinucleotide repeats of androgen receptor gene in male hepatocellular carcinoma". Int. J. Cancer 120 (8): 1610–7. April 2007. doi:10.1002/ijc.22479. PMID 17230529.
- ↑ "Androgen receptor gene polyglutamine length is associated with testicular histology in infertile patients". J. Urol. 169 (1): 224–7. January 2003. doi:10.1016/s0022-5347(05)64073-6. PMID 12478141.
- ↑ "Linkage between male infertility and trinucleotide repeat expansion in the androgen-receptor gene". Lancet 354 (9179): 640–3. August 1999. doi:10.1016/S0140-6736(98)08413-X. PMID 10466666.
- ↑ "Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility". J. Clin. Endocrinol. Metab. 82 (11): 3777–82. November 1997. doi:10.1210/jcem.82.11.4385. PMID 9360540.
- ↑ "Longer polyglutamine tracts in the androgen receptor are associated with moderate to severe undermasculinized genitalia in XY males". Hum. Mol. Genet. 9 (5): 829–34. March 2000. doi:10.1093/hmg/9.5.829. PMID 10749991.
- ↑ 46.0 46.1 "Significance of mutations in the androgen receptor gene in males with idiopathic infertility". J. Clin. Endocrinol. Metab. 85 (8): 2810–5. August 2000. doi:10.1210/jcem.85.8.6713. PMID 10946887.
- ↑ "Association of oestrogen receptor alpha polymorphisms and androgen receptor CAG trinucleotide repeats with male infertility: a study in 109 Greek infertile men". Int. J. Androl. 25 (3): 149–52. June 2002. doi:10.1046/j.1365-2605.2002.00339.x. PMID 12031042.
- ↑ "Inverse correlation between sperm concentration and number of androgen receptor CAG repeats in normal men". J. Clin. Endocrinol. Metab. 86 (6): 2585–90. June 2001. doi:10.1210/jcem.86.6.7608. PMID 11397858.
- ↑ "CAG repeat length in androgen-receptor gene and reproductive variables in fertile and infertile men". Lancet 359 (9300): 44–6. January 2002. doi:10.1016/S0140-6736(02)07280-X. PMID 11809188.
- ↑ "Male infertility and increased risk of diseases in future generations". Lancet 354 (9193): 1907–8. November 1999. doi:10.1016/S0140-6736(05)76874-4. PMID 10584751.
- ↑ "Hypospadias and the androgen receptor gene: mutation screening and CAG repeat length analysis". Mol. Hum. Reprod. 7 (5): 409–13. May 2001. doi:10.1093/molehr/7.5.409. PMID 11331662.
- ↑ "Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients". J. Androl. 28 (1): 164–9. 2007. doi:10.2164/jandrol.106.000927. PMID 16957138.
- ↑ "Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene". J. Clin. Endocrinol. Metab. 89 (10): 5105–9. October 2004. doi:10.1210/jc.2004-0293. PMID 15472213.
- ↑ "No association of androgen receptor GGN repeat length polymorphism with infertility in Indian men". J. Androl. 27 (6): 785–9. 2006. doi:10.2164/jandrol.106.000166. PMID 16809273.
- ↑ 55.0 55.1 "Pregnancy after hormonal correction of severe spermatogenic defect due to mutation in androgen receptor gene". Lancet 344 (8925): 826–7. September 1994. doi:10.1016/S0140-6736(94)92385-X. PMID 7993455.
- ↑ "Consensus statement on management of intersex disorders". Arch. Dis. Child. 91 (7): 554–63. July 2006. doi:10.1136/adc.2006.098319. PMID 16624884.
- ↑ 57.0 57.1 "Response to androgen treatment in a patient with partial androgen insensitivity and a mutation in the deoxyribonucleic acid-binding domain of the androgen receptor". J. Clin. Endocrinol. Metab. 83 (4): 1173–6. April 1998. doi:10.1210/jcem.83.4.4704. PMID 9543136.
- ↑ "Correlation of clinical, endocrine and molecular abnormalities with in vivo responses to high-dose testosterone in patients with partial androgen insensitivity syndrome". Clin. Endocrinol. 46 (4): 497–506. April 1997. doi:10.1046/j.1365-2265.1997.1140927.x. PMID 9196614.
- ↑ Nieschlag E (September 2006). "Testosterone treatment comes of age: new options for hypogonadal men". Clin. Endocrinol. 65 (3): 275–81. doi:10.1111/j.1365-2265.2006.02618.x. PMID 16918944.
External links
| Classification | |
|---|---|
| External resources |
 |

