Chemistry:Triethyl phosphite
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Triethyl phosphite | |
| Other names
Triethoxyphosphine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H15O3P | |
| Molar mass | 166.157 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.969 g/mL |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 156 °C (313 °F; 429 K) (57 to 58 °C at 16 mm) |
| Solubility | soluble in most organic solvents |
| -104.8·10−6 cm3/mol | |
| Hazards | |
| Main hazards | toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triethyl phosphite is an organophosphorus compound, specifically a phosphite ester, with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis.
The molecule features a pyramidal phosphorus(III) center bound to three ethoxide groups. Its 31P NMR spectrum features a signal at around +139 ppm vs phosphoric acid standard.
Triethylphosphite is prepared by treating phosphorus trichloride with ethanol in the presence of a base, typically a tertiary amine:[1]
- PCl3 + 3 EtOH + 3 R3N → P(OEt)3 + 3 R3NH + 3 Cl−
In the absence of the base, the reaction of ethanol and phosphorus trichloride affords diethylphosphite ((EtO)2P(O)H). Of the many related compounds can be prepared similarly, triisopropyl phosphite is an example (b.p. 43.5 °C/1.0 mm; CAS# 116-17-6).
Reactions
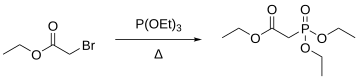
Triethyl phosphite can react with electrophiles in a Michaelis–Arbuzov reaction to produce organophosphonates. For example, the reaction between triethyl phosphite and ethyl bromoacetate produces a phosphonate suitable for use in the Horner–Wadsworth–Emmons reaction.[2]
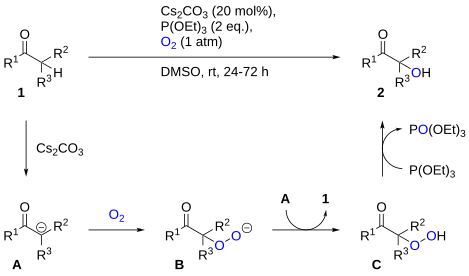
Reduction/deoxygenation of hydroperoxides to the alcohols can also be effected using triethyl phosphite.[2] This approach can be utilized for carbonyl α-hydroxylation by reacting the enolate with oxygen, producing an α-hydroperoxide which can be reduced by triethyl phosphite to the alcohol.[3] A proposed mechanism is shown below.[4]
Triethyl phosphite can also be used in the Corey–Winter olefin synthesis.[2]
As a ligand
In coordination chemistry and homogeneous catalysis, triethylphosphite finds use as a soft ligand. Its complexes are generally lipophilic and feature metals in low oxidation states. Examples include the colorless complexes FeH2(P(OEt)3)4 and Ni(P(OEt)3)4 (m.p. 108 °C).[5] It also forms a stable complex with copper(I) iodide.[2]
References
- ↑ Ford-Moore, A. H.; Perry, B. J. (1951). "Triethyl Phosphite". Org. Synth. 31: 111. doi:10.15227/orgsyn.031.0111.
- ↑ 2.0 2.1 2.2 2.3 Piscopio, Anthony D.; Shakya, Sagar (2005). "Triethyl Phosphite". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt224.pub2.
- ↑ Gardner, J. N.; Carlon, F. E.; Gnoj, O. (1968). "One-step procedure for the preparation of tertiary α-ketols from the corresponding ketones". The Journal of Organic Chemistry 33 (8): 3294–3297. doi:10.1021/jo01272a055.
- ↑ Liang, Yu-Feng; Jiao, Ning (2014). "Highly Efficient C–H Hydroxylation of Carbonyl Compounds with Oxygen under Mild Conditions". Angewandte Chemie International Edition 53 (2): 548–552. doi:10.1002/anie.201308698.
- ↑ Ittel, Steven D. (1990). "Complexes of Nickel(0)". Inorganic Syntheses 28: 98–104. doi:10.1002/9780470132593.ch26. ISBN 978-0-470-13259-3.
External links
 |