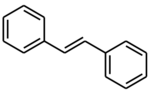
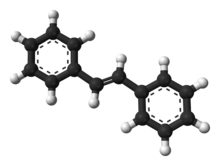
Chemistry:(E)-Stilbene
| |
| |
| Names | |
|---|---|
| IUPAC name
(E)-Stilbene[1]
| |
| Preferred IUPAC name
1,1′-[(E)-Ethene-1,2-diyl]dibenzene[1] | |
| Other names
Bibenzylidene
trans-α,β-Diphenylethylene (E)-1,2-Diphenylethylene ((1E)-2-Phenylvinyl)benzene trans-Stilbene [(E)-2-Phenylethenyl]benzene | |
| Identifiers | |
3D model (JSmol)
|
|
| 1616740 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 4381 | |
PubChem CID
|
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| C14H12 | |
| Molar mass | 180.250 g·mol−1 |
| Appearance | Solid |
| Density | 0.9707 g/cm3 |
| Melting point | 122 to 125 °C (252 to 257 °F; 395 to 398 K) |
| Boiling point | 305 to 307 °C (581 to 585 °F; 578 to 580 K) |
| Practically insoluble | |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
| Flash point | > 112 °C (234 °F; 385 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
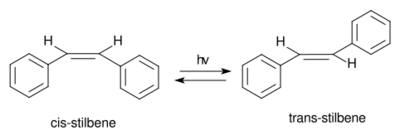
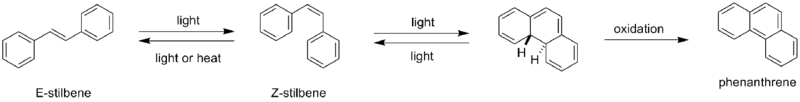
(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.
Stilbene was discovered in 1843 by the French chemist Auguste Laurent.[2] The name "stilbene" is derived from the Greek word στίλβω (stilbo), which means "I shine", on account of the lustrous appearance of the compound.[3]
Isomers
Stilbene exists as two possible stereoisomers. One is trans-1,2-diphenylethylene, called (E)-stilbene or trans-stilbene. The second is cis-1,2-diphenylethylene, called (Z)-stilbene or cis-stilbene, and is sterically hindered and less stable because the steric interactions force the aromatic rings out-of-plane and prevent conjugation.[4] Cis-stilbene is a liquid at room temperature (melting point: 5–6 °C (41–43 °F)), while trans-stilbene is a crystalline solid which does not melt until around 125 °C (257 °F), illustrating the two isomers have significantly different physical properties.[5][6]
Preparation and reactions
Many syntheses have been developed. One popular route entails reduction of benzoin using zinc amalgam.[6]
- C6H5–CH(OH)–C(=O)–C6H5 [math]\ce{ ->[\ce{Zn(Hg)}][\ce{HCl} \text{, } \ce{CH3CH2OH}] }[/math] trans-C6H5–CH=CH–C6H5
Both isomers of stilbene can be produced by decarboxylation of α-phenylcinnamic acid, trans-stilbene being produced from the (Z)-isomer of the acid.[5]
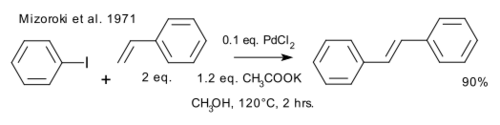
Richard F. Heck[7] and Tsutomu Mizoroki[8] independently reported the synthesis of trans-stilbene by coupling of iodobenzene and styrene using a palladium(II) catalyst, in what is now known as the Mizoroki-Heck reaction.[9][10] The Mizoroki approach produced the higher yield.
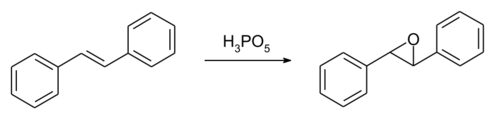
Stilbene undergoes reactions typical of alkenes. Trans-stilbene undergoes epoxidation with peroxymonophosphoric acid, H3PO5, producing a 74% yield of trans-stilbene oxide in dioxane.[11] The epoxide product formed is a racemic mixture of the two enantiomers of 1,2-diphenyloxirane. The achiral meso compound (1R,2S)-1,2-diphenyloxirane arises from cis-stilbene, though peroxide epoxidations of the cis-isomer produce both cis- and trans-epoxide products. For example, using tert-butyl hydroperoxide, oxidation of cis-stilbene produces 0.8% cis-stilbene oxide, 13.5% trans-stilbene oxide, and 6.1% benzaldehyde.[12][13] Enantiopure stilbene oxide has been prepared by Nobel laureate Karl Barry Sharpless.[14]
Stilbene can be cleanly oxidised to benzaldehyde by ozonolysis[15] or Lemieux–Johnson oxidation, and stronger oxidants such as acidified potassium permanganate will produce benzoic acid. Vicinal diols can be produced via the Upjohn dihydroxylation or enantioselectively using Sharpless asymmetric dihydroxylation[16][17] with enantiomeric excesses as high as 100%.[18][19][20]
Bromination of trans-stilbene produces predominantly meso-1,2-dibromo-1,2-diphenylethane (sometimes called meso-stilbene dibromide), in line with a mechanism involving a cyclic bromonium ion intermediate of a typical electrophilic bromine addition reaction;[21] cis-stilbene yields a racemic mixture of the two enantiomers of 1,2-dibromo-1,2-diphenylethane in a non-polar solvent such as carbon tetrachloride, but the extent of production of the meso compound increases with solvent polarity, with a yield of 90% in nitromethane.[22] The formation of small quantities of the two enantiomers of stilbene dibromide from the trans-isomer suggests that the bromonium ion intermediate exists in chemical equilibrium with a carbocation intermediate PhCHBr–C+(H)Ph with a vacant p orbital vulnerable to nucleophilic attack from either face.[21] The addition of bromide or tribromide salts restores much of the stereospecificity even in solvents with a dielectric constant above 35.[23]
Upon UV irradiation it converts to cis-stilbene, a classic example of a photochemical reaction involving trans-cis isomerization, and can undergo further reaction to form phenanthrene.[24]
Derivatives and uses
Synthetic
(E)-Stilbene itself is of little value, but it is a precursor to other derivatives used as dyes, optical brighteners, phosphors, and scintillators.[25] Stilbene is one of the gain mediums used in dye lasers.[26]
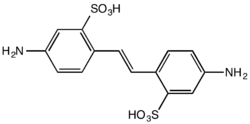
Disodium 4,4'-dinitrostilbene-2,2'-disulfonate is prepared by the sulfonation of 4-nitrotoluene to form 4-nitrotoluene-2-sulfonic acid, which can then be oxidatively coupled using sodium hypochlorite to form the (E)-stilbene derivative[27] in a process originally developed by Arthur George Green and André Wahl in the late nineteenth century.[28][29] Improvements to the process with higher yields have been developed, using air oxidation in liquid ammonia.[30] The product is useful as its reaction with aniline derivatives results in the formation of azo dyes. Commercially important dyes derived from this compound include Direct Red 76, Direct Brown 78, and Direct Orange 40.[26]
Natural stilbenes
The stilbenoids are naturally occurring stilbene derivatives. Examples include resveratrol and its cousin, pterostilbene. The stilbestrols, which are structurally but not synthetically related to (E)-stilbene, exhibit estrogenic activity. Members of this group include diethylstilbestrol, fosfestrol, and dienestrol. Some such derivative are produced by condensation of coenzyme A derivatives of cinnamic acid or 4-hydroxycinnamic acid and the malonic acid.
Appendix
Table 1. Vapor pressures[31]
| Isomer | Temperature, °C | Vapor pressure, kPa |
|---|---|---|
| cis-stilbene | 100 | 0.199 |
| cis-stilbene | 125 | 0.765 |
| cis-stilbene | 150 | 2.51 |
| trans-stilbene | 150 | 0.784 |
References
- ↑ 1.0 1.1 International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 379. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Laurent, Auguste (1843). "Mémoire sur la série stilbique" (in French). Comptes rendus 16: 856–860. https://books.google.com/books?id=hzEt49manA8C&pg=PA167. From p. 857: "En soumettant ce sulfure à la distillation, il donne plusieurs produits, et entre autres, un composé fort remarquable que je nomme stilbène." (On submitting this sulfide [i.e., phenyl thioaldehyde, C6H5(CS)H] to [dry] distillation, it gives several products, and among others, a very remarkable compound which I name "stilbene".)
- ↑ Miller, William Allen (1880). Elements of Chemistry: Theoretical and Practical. 3 (5th ed.). London, England: Longmans, Green and Co.. p. 366. https://books.google.com/books?id=H4kMAQAAIAAJ&pg=PA366.
- ↑ Eliel, Ernest L.; Wilen, Samuel H. (1994). Stereochemistry of Organic Compounds. John Wiley and Sons. pp. 566-567. ISBN 0-471-01670-5. https://archive.org/details/stereochemistryo0000elie_a9t3.
- ↑ 5.0 5.1 Buckles, Robert E.; Wheeler, Norris G. (1953). "cis-Stilbene". Organic Syntheses 33: 88. doi:10.15227/orgsyn.033.0088. http://www.orgsyn.org/demo.aspx?prep=CV4P0857.; Collective Volume, 4, pp. 857
- ↑ 6.0 6.1 Shriner, R. L.; Berger, Alfred (1943). "trans-Stilbene". Organic Syntheses 23: 86. doi:10.15227/orgsyn.023.0086. http://www.orgsyn.org/demo.aspx?prep=CV3P0786.; Collective Volume, 3, pp. 786
- ↑ Heck, R. F.; Nolley, J. P. (1972). "Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides". J. Org. Chem. 37 (14): 2320–2322. doi:10.1021/jo00979a024.
- ↑ Mizoroki, Tsutomu; Mori, Kunio; Ozaki, Atsumu (1971). "Arylation of Olefin with Aryl Iodide Catalyzed by Palladium". Bull. Chem. Soc. Jpn. 44 (2): 581. doi:10.1246/bcsj.44.581.
- ↑ Heck, Richard F. (1982). "Palladium-Catalyzed Vinylation of Organic Halides". Organic Reactions. 27. pp. 345–390. doi:10.1002/0471264180.or027.02. ISBN 0471264180.
- ↑ Beletskaya, Irina P.; Cheprakov, Andrei V. (2000). "The Heck Reaction as a Sharpening Stone of Palladium Catalysis". Chem. Rev. 100 (8): 3009–3066. doi:10.1021/cr9903048. PMID 11749313.
- ↑ Ogata, Yoshiro; Tomizawa, Kohtaro; Ikeda, Toshiyuki (1979). "Oxidation of trans-stilbene with peroxymonophosphoric acid". J. Org. Chem. 44 (14): 2362–2364. doi:10.1021/jo01328a006.
- ↑ Yin, Guochuan; Danby, Andrew M.; Kitko, David; Carter, John D.; Scheper, William M.; Busch, Daryle H. (2007). "Olefin Epoxidation by Alkyl Hydroperoxide with a Novel Cross-Bridged Cyclam Manganese Complex: Demonstration of Oxygenation by Two Distinct Reactive Intermediates". Inorg. Chem. 46 (6): 2173–2180. doi:10.1021/ic061957r. PMID 17295471.
- ↑ Busch, Daryle H.; Yin, Guochuan; Less, Hyun-Jin (2011). "Lewis Acid Catalyzed Epoxidation of Olefins Using Hydrogen Peroxide: Growing Prominence and Expanding Range". in Oyama, S. Ted. Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis. Elsevier. pp. 119–153. ISBN 9780080558011. https://books.google.com/books?id=xJpkegngTqAC&pg=PA143.
- ↑ Chang, Han-Ting; Sharpless, K. Barry (1996). "Molar Scale Synthesis of Enantiopure Stilbene Oxide". J. Org. Chem. 61 (18): 6456–6457. doi:10.1021/jo960718q. PMID 11667495.
- ↑ Bishop, Clyde E.; Denson, Donald D.; Story, Paul R. (1968). "Mechanisms of ozonolysis. The cis, trans-stilbene system". Tetrahedron Lett. 9 (55): 5739–5742. doi:10.1016/S0040-4039(00)76338-6.
- ↑ Jacobsen, Eric N.; Marko, Istvan; Mungall, William S.; Schroeder, Georg; Sharpless, K. Barry (1988). "Asymmetric dihydroxylation via ligand-accelerated catalysis". J. Am. Chem. Soc. 110 (6): 1968–1970. doi:10.1021/ja00214a053.
- ↑ Kolb, Hartmuth C.; VanNieuwenhze, Michael S.; Sharpless, K. Barry (1994). "Catalytic Asymmetric Dihydroxylation". Chem. Rev. 94 (8): 2483–2547. doi:10.1021/cr00032a009.
- ↑ Wang, Zhi-Min; Sharpless, K. Barry (1994). "A Solid-to-Solid Asymmetric Dihydroxylation Procedure for Kilogram-Scale Preparation of Enantiopure Hydrobenzoin". J. Org. Chem. 59 (26): 8302–8303. doi:10.1021/jo00105a065.
- ↑ McKee, Blaine H.; Gilheany, Declan G.; Sharpless, K. Barry (1992). "(R,R)-1,2-Diphenyl-1,2-ethanediol (Stilbene Diol)". Organic Syntheses 70: 47. doi:10.15227/orgsyn.070.0047. http://www.orgsyn.org/demo.aspx?prep=CV9P0383.; Collective Volume, 9, pp. 383
- ↑ Atta-ur-Rahman; Shah, Zahir (1993). "Asymmetric Hydroxylations". Stereoselectove Synthesis in Organic Chemistry. Springer-Verlag. pp. 406–410. ISBN 9781461383277. https://books.google.com/books?id=BJfbBwAAQBAJ&pg=PA408.
- ↑ 21.0 21.1 Gilbert, John C.; Martin, Stephen F. (2010). "10.6 – Bromination of Alkenes". Experimental Organic Chemistry: A Miniscale and Microscale Approach (5th ed.). Cengage Learning. pp. 376–383. ISBN 9781439049143. https://books.google.com/books?id=8wIQwCmWz9EC&pg=PA376.
- ↑ Buckles, Robert E.; Bader, Jane M.; Thurmaier, Roland J. (1962). "Stereospecificity of the Addition of Bromine to cis- and trans-Stilbene". J. Org. Chem. 27 (12): 4523–4527. doi:10.1021/jo01059a097.
- ↑ Bianchini, Roberto; Chiappe, Cinzia (1992). "Stereoselectivity and reversibility of electrophilic bromine addition to stilbenes in chloroform: influence of the bromide-tribromide-pentabromide equilibrium in the counteranion of the ionic intermediates". J. Org. Chem. 57 (24): 6474–6478. doi:10.1021/jo00050a021.
- ↑ Kwasniewski, S. P.; Claes, L.; François, J.-P.; Deleuze, M. S. (2003). "High level theoretical study of the structure and rotational barriers of trans-stilbene". J. Chem. Phys. 118 (17): 7823–7836. doi:10.1063/1.1563617. Bibcode: 2003JChPh.118.7823K.
- ↑ Vogt, Peter F.; Gerulis, John J. (2000). "Amines, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_037. ISBN 3527306730.
- ↑ 26.0 26.1 Hunger, Klaus; Mischke, Peter; Rieper, Wolfgang; Raue, Roderich; Kunde, Klaus; Engel, Aloys (2000). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245. ISBN 3527306730.
- ↑ Cumming, William M.; Hopper, I. Vance; Wheeler, T. Sherlock (1926). "Preparation 294.—Dinitro-Stilbene-Disulphonic Acid (Na salt)". Systematic Organic Chemistry: Modern Methods of Preparation and Estimation. New York: D. Van Nostrand Company. p. 314. https://archive.org/details/systematicorgani00cumm_0.
- ↑ Green, Arthur G.; Wahl, André R. (1897). "Ueber die Oxydation von Paranitrotoluolsulfosäure" (in German). Ber. Dtsch. Chem. Ges. 30 (3): 3097–3101. doi:10.1002/cber.189703003128. https://zenodo.org/record/1425882.
- ↑ Green, Arthur G.; Wahl, André R. (1898). "Ueber die Oxydation der Paranitrotoluolsulfosäure" (in German). Ber. Dtsch. Chem. Ges. 31 (1): 1078–1080. doi:10.1002/cber.189803101195. https://zenodo.org/record/1425898.
- ↑ Guglielmetti, Leonardo, "Process for the preparation of 4,4'-dinitrostilbene-2,2-disulfonic acid", US patent 5041632, published 1991-08-20, issued 1991-08-20, assigned to Ciba-Geigy Corporation
- ↑ Lide, David (1995). CRC Handbook of Chemistry and Physics (76th ed.). USA: CRC Press, Inc.. pp. 6–107. ISBN 0-8493-0476-8.
External links
 |