Chemistry:Dienestrol
 | |
| Clinical data | |
|---|---|
| Trade names | Ortho Dienestrol, Dienoestrol, Dienoestrol Ortho, Sexadien, Cycladiene, Denestrolin, Dienol, Dinovex, Follormon, Oestrodiene, Synestrol |
| Other names | Dienoestrol; p-[(E,E)-1-Ethylidene-2-(p-hydroxyphenyl)-2-butenyl]phenol; 3,4-Di(para-hydroxyphenyl)-2,4-hexadiene |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Drug class | Nonsteroidal estrogen |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H18O2 |
| Molar mass | 266.340 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
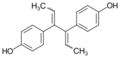
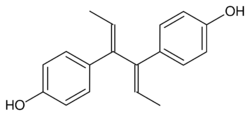
Dienestrol (INN, USAN) (brand names Dienoestrol, Denestrolin, Dienol and many others[lower-alpha 1]), also known as dienoestrol (BAN), is a synthetic nonsteroidal estrogen medication of the stilbestrol group which is or was used to treat menopausal symptoms in the United States and Europe.[1][2][3][4] It has been studied for use by rectal administration in the treatment of prostate cancer in men as well.[5] The medication was introduced in the U.S. in 1947 by Schering as Synestrol and in France in 1948 as Cycladiene.[4] Dienestrol is a close analogue of diethylstilbestrol.[6] It has approximately 223% and 404% of the affinity of estradiol at the ERα and ERβ, respectively.[7]
Dienestrol diacetate (brand names Faragynol, Gynocyrol, others) also exists and has been used medically.[2]
Isomers
-
Dienestrol (unspecified) - CAS 84-17-3
-
E,E-Dienestrol - CAS 13029-44-2
-
Z,Z-Dienestrol - CAS 35495-11-5
See also
Notes
- ↑ Other trade names of the medication include Ortho Dienestrol, Dienoestrol Ortho, Sexadien, Dinovex, Follormon, Oestrodiene, and Synestrol
References
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 331–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA331.
- ↑ 2.0 2.1 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 390–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA390.
- ↑ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 361–. ISBN 978-3-7692-2114-5. https://books.google.com/books?id=2HBPHmclMWIC&pg=PA361.
- ↑ 4.0 4.1 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1286–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1286.
- ↑ "Somministrazione degli estrogeni per via rettale nel carcinoma prostatico." (in it). Minerva Urol 5 (1): 28–32. 1953. ISSN 0026-4989. PMID 13063334.
- ↑ VITAMINS AND HORMONES. Academic Press. 1 January 1945. pp. 233–. ISBN 978-0-08-086600-0. https://archive.org/details/in.ernet.dli.2015.5563.
- ↑ "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology 138 (3): 863–70. 1997. doi:10.1210/endo.138.3.4979. PMID 9048584.
 |