Chemistry:Molecular self-assembly
Template:Molecular self-assembly subfields
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intermolecular and intramolecular. Commonly, the term molecular self-assembly refers to the former, while the latter is more commonly called folding.
Supramolecular systems
Molecular self-assembly is a key concept in supramolecular chemistry.[6][7][8] This is because assembly of molecules in such systems is directed through non-covalent interactions (e.g., hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi-stacking interactions, and/or electrostatic) as well as electromagnetic interactions. Common examples include the formation of colloids, biomolecular condensates, micelles, vesicles, liquid crystal phases, and Langmuir monolayers by surfactant molecules.[9] Further examples of supramolecular assemblies demonstrate that a variety of different shapes and sizes can be obtained using molecular self-assembly.[10]
Molecular self-assembly allows the construction of challenging molecular topologies. One example is Borromean rings, interlocking rings wherein removal of one ring unlocks each of the other rings. DNA has been used to prepare a molecular analog of Borromean rings.[11] More recently, a similar structure has been prepared using non-biological building blocks.[12]
Biological systems
Molecular self-assembly underlies the construction of biologic macromolecular assemblies and biomolecular condensates in living organisms, and so is crucial to the function of cells. It is exhibited in the self-assembly of lipids to form the membrane, the formation of double helical DNA through hydrogen bonding of the individual strands, and the assembly of proteins to form quaternary structures. Molecular self-assembly of incorrectly folded proteins into insoluble amyloid fibers is responsible for infectious prion-related neurodegenerative diseases. Molecular self-assembly of nanoscale structures plays a role in the growth of the remarkable β-keratin lamellae/setae/spatulae structures used to give geckos the ability to climb walls and adhere to ceilings and rock overhangs.[13][14]
Protein multimers
When multiple copies of a polypeptide encoded by a gene self-assemble to form a complex, this protein structure is referred to as a "multimer".[15] Genes that encode multimer-forming polypeptides appear to be common. When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. In such a case, the phenomenon is referred to as intragenic complementation.[16] Jehle pointed out that, when immersed in a liquid and intermingled with other molecules, charge fluctuation forces favor the association of identical molecules as nearest neighbors.[17]
Nanotechnology
Molecular self-assembly is an important aspect of bottom-up approaches to nanotechnology. Using molecular self-assembly, the final (desired) structure is programmed in the shape and functional groups of the molecules. Self-assembly is referred to as a 'bottom-up' manufacturing technique in contrast to a 'top-down' technique such as lithography where the desired final structure is carved from a larger block of matter. In the speculative vision of molecular nanotechnology, microchips of the future might be made by molecular self-assembly. An advantage to constructing nanostructure using molecular self-assembly for biological materials is that they will degrade back into individual molecules that can be broken down by the body.
DNA nanotechnology
DNA nanotechnology is an area of current research that uses the bottom-up, self-assembly approach for nanotechnological goals. DNA nanotechnology uses the unique molecular recognition properties of DNA and other nucleic acids to create self-assembling branched DNA complexes with useful properties.[18] DNA is thus used as a structural material rather than as a carrier of biological information, to make structures such as complex 2D and 3D lattices (both tile-based as well as using the "DNA origami" method) and three-dimensional structures in the shapes of polyhedra.[19] These DNA structures have also been used as templates in the assembly of other molecules such as gold nanoparticles[20] and streptavidin proteins.[21]
Two-dimensional monolayers
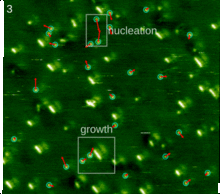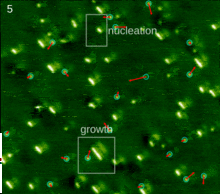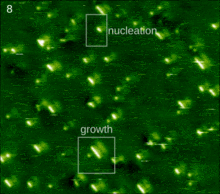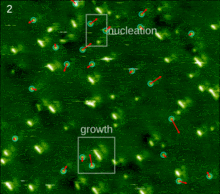
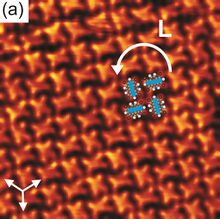
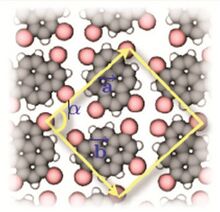
The spontaneous assembly of a single layer of molecules at interfaces is usually referred to as two-dimensional self-assembly. One of the common examples of such assemblies are Langmuir-Blodgett monolayers and multilayers of surfactants. Non-surface active molecules can assemble into ordered structures as well. Early direct proofs showing that non-surface active molecules can assemble into higher-order architectures at solid interfaces came with the development of scanning tunneling microscopy and shortly thereafter.[22] Eventually two strategies became popular for the self-assembly of 2D architectures, namely self-assembly following ultra-high-vacuum deposition and annealing and self-assembly at the solid-liquid interface.[23] The design of molecules and conditions leading to the formation of highly-crystalline architectures is considered today a form of 2D crystal engineering at the nanoscopic scale.
See also
- Assembly theory
- Foldamer
- Ice-nine
- Macromolecular assembly
- Self-assembly of nanoparticles
- Supramolecular assembly
References
- ↑ Sweetman, A. M.; Jarvis, S. P.; Sang, Hongqian; Lekkas, I.; Rahe, P.; Wang, Yu; Wang, Jianbo; Champness, N.R. et al. (2014). "Mapping the force field of a hydrogen-bonded assembly". Nature Communications 5: 3931. doi:10.1038/ncomms4931. PMID 24875276. Bibcode: 2014NatCo...5.3931S.
- ↑ Hapala, Prokop; Kichin, Georgy; Wagner, Christian; Tautz, F. Stefan; Temirov, Ruslan; Jelínek, Pavel (2014-08-19). "Mechanism of high-resolution STM/AFM imaging with functionalized tips". Physical Review B 90 (8): 085421. doi:10.1103/PhysRevB.90.085421. Bibcode: 2014PhRvB..90h5421H.
- ↑ Hämäläinen, Sampsa K.; van der Heijden, Nadine; van der Lit, Joost; den Hartog, Stephan; Liljeroth, Peter; Swart, Ingmar (2014-10-31). "Intermolecular Contrast in Atomic Force Microscopy Images without Intermolecular Bonds". Physical Review Letters 113 (18): 186102. doi:10.1103/PhysRevLett.113.186102. PMID 25396382. Bibcode: 2014PhRvL.113r6102H.
- ↑ Kling, Felix (2016). Diffusion and structure formation of molecules on calcite(104) (PhD). Johannes Gutenberg-Universität Mainz. doi:10.25358/openscience-2179.
- ↑ Pham, Tuan Anh; Song, Fei; Nguyen, Manh-Thuong; Stöhr, Meike (2014). "Self-assembly of pyrene derivatives on Au(111): Substituent effects on intermolecular interactions". Chem. Commun. 50 (91): 14089–14092. doi:10.1039/C4CC02753A. PMID 24905327.
- ↑ Lehn, J.-M. (1988). "Perspectives in Supramolecular Chemistry-From Molecular Recognition towards Molecular Information Processing and Self-Organization". Angew. Chem. Int. Ed. Engl. 27 (11): 89–121. doi:10.1002/anie.198800891.
- ↑ Lehn, J.-M. (1990). "Supramolecular Chemistry-Scope and Perspectives: Molecules, Supermolecules, and Molecular Devices (Nobel Lecture)". Angew. Chem. Int. Ed. Engl. 29 (11): 1304–1319. doi:10.1002/anie.199013041.
- ↑ Lehn, J.-M.. Supramolecular Chemistry: Concepts and Perspectives. Wiley-VCH. ISBN 978-3-527-29311-7.
- ↑ Rosen, Milton J. (2004). Surfactants and interfacial phenomena. Hoboken, NJ: Wiley-Interscience. ISBN 978-0-471-47818-8.
- ↑ Ariga, Katsuhiko; Hill, Jonathan P; Lee, Michael V; Vinu, Ajayan; Charvet, Richard; Acharya, Somobrata (2008). "Challenges and breakthroughs in recent research on self-assembly". Science and Technology of Advanced Materials 9 (1): 014109. doi:10.1088/1468-6996/9/1/014109. PMID 27877935. Bibcode: 2008STAdM...9a4109A.
- ↑ Mao, C; Sun, W; Seeman, N. C. (1997). "Assembly of Borromean rings from DNA". Nature 386 (6621): 137–138. doi:10.1038/386137b0. PMID 9062186. Bibcode: 1997Natur.386..137M.
- ↑ Chichak, K. S.; Cantrill, S. J.; Pease, A. R.; Chiu, S. H.; Cave, G. W.; Atwood, J. L.; Stoddart, J. F. (2004). "Molecular Borromean Rings". Science 304 (5675): 1308–1312. doi:10.1126/science.1096914. PMID 15166376. Bibcode: 2004Sci...304.1308C. http://irep.ntu.ac.uk/id/eprint/22968/1/196491_534%20Cave%20PostPrint.pdf.
- ↑ Min, Younjin (2008). "The role of interparticle and external forces in nanoparticle assembly". Nature Materials 7 (7): 527–38. doi:10.1038/nmat2206. PMID 18574482. Bibcode: 2008NatMa...7..527M.
- ↑ Santos, Daniel; Spenko, Matthew; Parness, Aaron; Kim, Sangbae; Cutkosky, Mark (2007). "Directional adhesion for climbing: theoretical and practical considerations". Journal of Adhesion Science and Technology 21 (12–13): 1317–1341. doi:10.1163/156856107782328399. "Gecko "feet and toes are a hierarchical system of complex structures consisting of lamellae, setae, and spatulae. The distinguishing characteristics of the gecko adhesion system have been described [as] (1) anisotropic attachment, (2) high pulloff force to preload ratio, (3) low detachment force, (4) material independence, (5) self-cleaning, (6) anti-self sticking and (7) non-sticky default state. ... The gecko’s adhesive structures are made from ß-keratin (modulus of elasticity [approx.] 2 GPa). Such a stiff material is not inherently sticky; however, because of the gecko adhesive’s hierarchical nature and extremely small distal features (spatulae are [approx.] 200 nm in size), the gecko’s foot is able to intimately conform to the surface and generate significant attraction using van der Waals forces.".
- ↑ Crick FH, Orgel LE. The theory of inter-allelic complementation. J Mol Biol. 1964 Jan;8:161-5. doi: 10.1016/s0022-2836(64)80156-x. PMID: 14149958
- ↑ Bernstein H, Edgar RS, Denhardt GH. Intragenic complementation among temperature sensitive mutants of bacteriophage T4D. Genetics. 1965;51(6):987-1002.
- ↑ H. Jehle (1963), "Intermolecular forces and biological specificity", Proc Natl Acad Sci USA 50 (3): 516–524, doi:10.1073/pnas.50.3.516, PMID 16578546
- ↑ Seeman, N. C. (2003). "DNA in a material world". Nature 421 (6921): 427–431. doi:10.1038/nature01406. PMID 12540916. Bibcode: 2003Natur.421..427S.
- ↑ Chen, J.; Seeman, N. C. (1991). "Synthesis from DNA of a molecule with the connectivity of a cube". Nature 350 (6319): 631–633. doi:10.1038/350631a0. PMID 2017259. Bibcode: 1991Natur.350..631C.
- ↑ Mirkin, C. A.; Letsinger, R. L.; Mucic, R. C.; Storhoff, J. J. (1996). "A DNA-based method for rationally assembling nanoparticles into macroscopic materials". Nature 382 (6592): 607–609. doi:10.1038/382607a0. PMID 8757129. Bibcode: 1996Natur.382..607M.
- ↑ Yan, H; Park, S. H.; Finkelstein, G; Reif, J. H.; Labean, T. H. (2003). "DNA-Templated Self-Assembly of Protein Arrays and Highly Conductive Nanowires". Science 301 (5641): 1882–1884. doi:10.1126/science.1089389. PMID 14512621. Bibcode: 2003Sci...301.1882Y.
- ↑ Foster, J. S.; Frommer, J. E. (1988). "Imaging of liquid crystals using a tunnelling microscope". Nature 333 (6173): 542–545. doi:10.1038/333542a0. Bibcode: 1988Natur.333..542F.
- ↑ Rabe, J.P.; Buchholz, S. (1991). "Commensurability and Mobility in Two-Dimensional Molecular Patterns on Graphite". Science 253 (5018): 424–427. doi:10.1126/science.253.5018.424. PMID 17746397. Bibcode: 1991Sci...253..424R.
ar:تجميع ذاتي جزيئي
 |