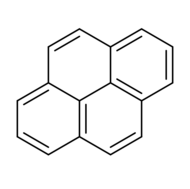
Chemistry:Pyrene
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Pyrene[1] | |
| Other names
Benzo[def]phenanthrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1307225 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 84203 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C16H10 | |
| Molar mass | 202.256 g·mol−1 |
| Appearance | colorless solid
(yellow impurities are often found at trace levels in many samples). |
| Density | 1.271 g/cm3[2] |
| Melting point | 150.62 °C (303.12 °F; 423.77 K)[2] |
| Boiling point | 394 °C (741 °F; 667 K)[2] |
| 0.049 mg/L (0 °C) 0.139 mg/L (25 °C) 2.31 mg/L (75 °C)[3] | |
| log P | 5.08[4] |
| Band gap | 2.02 eV[5] |
| -147·10−6 cm3/mol[6] | |
| Structure[7] | |
| Monoclinic | |
| P21/a | |
a = 13.64 Å, b = 9.25 Å, c = 8.47 Å α = 90°, β = 100.28°, γ = 90°
| |
Formula units (Z)
|
4 |
| Thermochemistry[8] | |
Heat capacity (C)
|
229.7 J/(K·mol) |
Std molar
entropy (S |
224.9 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
125.5 kJ·mol−1 |
Enthalpy of fusion (ΔfH⦵fus)
|
17.36 kJ·mol−1 |
| Hazards | |
| Main hazards | irritant |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Related PAHs
|
benzopyrene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C
16H
10. This yellow-green solid is the smallest peri-fused PAH (one where the rings are fused through more than one face). Pyrene forms during incomplete combustion of organic compounds.[10]
Occurrence and properties
Pyrene was first isolated from coal tar, where it occurs up to 2% by weight. As a peri-fused PAH, pyrene is much more resonance-stabilized than its five-member-ring containing isomer fluoranthene. Therefore, it is produced in a wide range of combustion conditions. For example, automobiles produce about 1 μg/km.[11]
Reactions
Oxidation with chromate affords perinaphthenone and then naphthalene-1,4,5,8-tetracarboxylic acid. Pyrene undergoes a series of hydrogenation reactions and is susceptible to halogenation, Diels-Alder additions, and nitration, all with varying degrees of selectivity.[11] Bromination occurs at one of the 3-positions.[12]
Reduction with sodium affords the radical anion. From this anion, a variety of pi-arene complexes can be prepared.[13]
Photophysics
Pyrene and its derivatives are used commercially to make dyes and dye precursors, for example pyranine and naphthalene-1,4,5,8-tetracarboxylic acid. It has strong absorbance in UV-Vis in three sharp bands at 330 nm in DCM. The emission is close to the absorption, but moving at 375 nm.[14] The morphology of the signals change with the solvent. Its derivatives are also valuable molecular probes via fluorescence spectroscopy, having a high quantum yield and lifetime (0.65 and 410 nanoseconds, respectively, in ethanol at 293 K). Pyrene was the first molecule for which excimer behavior was discovered.[15] Such excimer appears around 450 nm. Theodor Förster reported this in 1954.[16]
Applications
Pyrene's fluorescence emission spectrum is very sensitive to solvent polarity, so pyrene has been used as a probe to determine solvent environments. This is due to its excited state having a different, non-planar structure than the ground state. Certain emission bands are unaffected, but others vary in intensity due to the strength of interaction with a solvent.
Pyrenes are strong electron donor materials and can be combined with several materials in order to make electron donor-acceptor systems which can be used in energy conversion and light harvesting applications.[14]
Safety and environmental factors
Although it is not as problematic as benzopyrene, animal studies have shown pyrene is toxic to the kidneys and liver. It is now known that pyrene affects several living functions in fish and algae.[18][19][20][21]
Its biodegradation has been heavily examined. The process commences with dihydroxylation at each of two kinds of CH=CH linkages.[22] Experiments in pigs show that urinary 1-hydroxypyrene is a metabolite of pyrene, when given orally.[23]
See also
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 206. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 Haynes, p. 3.472
- ↑ Haynes, p. 5.162
- ↑ Haynes, p. 5.176
- ↑ Haynes, p. 12.96
- ↑ Haynes, p. 3.579
- ↑ Camerman, A.; Trotter, J. (1965). "The crystal and molecular structure of pyrene". Acta Crystallographica 18 (4): 636–643. doi:10.1107/S0365110X65001494.
- ↑ Haynes, pp. 5.34, 6.161
- ↑ GHS: PubChem
- ↑ Figueira-Duarte, Teresa M.; Müllen, Klaus (2011). "Pyrene-Based Materials for Organic Electronics". Chemical Reviews 111 (11): 7260–7314. doi:10.1021/cr100428a. PMID 21740071.
- ↑ 11.0 11.1 Senkan, Selim and Castaldi, Marco (2003) "Combustion" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim.
- ↑ Gumprecht, W. H. (1968). "3-Bromopyrene". Org. Synth. 48: 30. doi:10.15227/orgsyn.048.0030.
- ↑ Kucera, Benjamin E.; Jilek, Robert E.; Brennessel, William W.; Ellis, John E. (2014). "Bis(pyrene)metal complexes of vanadium, niobium and titanium: Isolable homoleptic pyrene complexes of transition metals". Acta Crystallographica Section C: Structural Chemistry 70 (8): 749–753. doi:10.1107/S2053229614015290. PMID 25093352.
- ↑ 14.0 14.1 Tagmatarchis, Nikos; Ewels, Christopher P.; Bittencourt, Carla; Arenal, Raul; Pelaez-Fernandez, Mario; Sayed-Ahmad-Baraza, Yuman; Canton-Vitoria, Ruben (2017-06-05). "Functionalization of MoS 2 with 1,2-dithiolanes: toward donor-acceptor nanohybrids for energy conversion" (in en). npj 2D Materials and Applications 1 (1): 13. doi:10.1038/s41699-017-0012-8. ISSN 2397-7132.
- ↑ Van Dyke, David A.; Pryor, Brian A.; Smith, Philip G.; Topp, Michael R. (May 1998). "Nanosecond Time-Resolved Fluorescence Spectroscopy in the Physical Chemistry Laboratory: Formation of the Pyrene Excimer in Solution". Journal of Chemical Education 75 (5): 615. doi:10.1021/ed075p615. Bibcode: 1998JChEd..75..615V.
- ↑ Förster, Th.; Kasper, K. (June 1954). "Ein Konzentrationsumschlag der Fluoreszenz.". Zeitschrift für Physikalische Chemie 1 (5_6): 275–277. doi:10.1524/zpch.1954.1.5_6.275.
- ↑ Pham, Tuan Anh; Song, Fei; Nguyen, Manh-Thuong; Stöhr, Meike (2014). "Self-assembly of pyrene derivatives on Au(111): Substituent effects on intermolecular interactions". Chem. Commun. 50 (91): 14089–92. doi:10.1039/C4CC02753A. PMID 24905327.
- ↑ Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. (2013). "Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae)". Ecological Indicators 34: 641–647. doi:10.1016/j.ecolind.2013.06.019.
- ↑ Oliveira, M.; Gravato, C.; Guilhermino, L. (2012). "Acute toxic effects of pyrene on Pomatoschistus microps (Teleostei, Gobiidae): Mortality, biomarkers and swimming performance". Ecological Indicators 19: 206–214. doi:10.1016/j.ecolind.2011.08.006.
- ↑ Oliveira, M.; Ribeiro, A.; Guilhermino, L. (2012). "Effects of exposure to microplastics and PAHs on microalgae Rhodomonas baltica and Tetraselmis chuii". Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 163: S19–S20. doi:10.1016/j.cbpa.2012.05.062.
- ↑ Oliveira, M.; Ribeiro, A.; Guilhermino, L. (2012). "Effects of short-term exposure to microplastics and pyrene on Pomatoschistus microps (Teleostei, Gobiidae)". Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 163: S20. doi:10.1016/j.cbpa.2012.05.063.
- ↑ Seo, Jong-Su; Keum, Young-Soo; Li, Qing (2009). "Bacterial Degradation of Aromatic Compounds". International Journal of Environmental Research and Public Health 6 (1): 278–309. doi:10.3390/ijerph6010278. PMID 19440284.
- ↑ Keimig, S. D.; Kirby, K. W.; Morgan, D. P.; Keiser, J. E.; Hubert, T. D. (1983). "Identification of 1-hydroxypyrene as a major metabolite of pyrene in pig urine". Xenobiotica 13 (7): 415–20. doi:10.3109/00498258309052279. PMID 6659544.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
Further reading
- Birks, J. B. (1969). Photophysics of Aromatic Molecules. London: Wiley.
- Valeur, B. (2002). Molecular Fluorescence: Principles and Applications. New York: Wiley-VCH.
- Birks, J. B. (1975). "Excimers" (in en). Reports on Progress in Physics 38 (8): 903–974. doi:10.1088/0034-4885/38/8/001. ISSN 0034-4885. Bibcode: 1975RPPh...38..903B.
- Fetzer, J. C. (2000). The Chemistry and Analysis of the Large Polycyclic Aromatic Hydrocarbons. New York: Wiley.
 |




