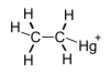
Chemistry:Ethylmercury
|
| |||
| Identifiers | |||
|---|---|---|---|
| |||
3D model (JSmol)
|
| ||
| 3903035 | |||
| ChEBI |
| ||
| ChemSpider | |||
| EC Number |
| ||
| 323460 | |||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| C2H5Hg+ | |||
| Molar mass | 229.65 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ethylmercury (sometimes ethyl mercury) is a cation composed of an organic CH3CH2- species (an ethyl group) bound to a mercury(II) centre, making it a type of organometallic cation, and giving it a chemical formula C2H5Hg+. The main source of ethylmercury is thimerosal.[1]
Synthesis and structure
Ethylmercury (C2H5Hg+) is a substituent of compounds: it occurs as a component of compounds of the formula C2H5HgX where X = chloride, thiolate, or another organic group. Most famously X = the mercaptide group of thiosalicylic acid as in thiomersal. In the body, ethylmercury is most commonly encountered as derivatives with a thiolate attached to the mercury.[2] In these compounds, Hg(II) has a linear or sometimes trigonal coordination geometry. Given the comparable electronegativities of mercury and carbon, the mercury-carbon bond is described as covalent.[3]:p. 79
Toxicity
The toxicity of ethylmercury is well studied.[4][1] Like methylmercury, ethylmercury distributes to all body tissues, crossing the blood–brain barrier and the placental barrier, and ethylmercury also moves freely throughout the body.[5] Risk assessment for effects on the human nervous system have been made by extrapolating from dose-response relationships for methylmercury.[1] Estimates have suggested that ethylmercury clears from blood with a half-life of 3–7 days in adult humans; however, this area has not been well studied.[6][7]
Public health concerns
Concerns based on extrapolations of the effect of methylmercury caused thimerosal to be removed from U.S. childhood vaccines in 1999, but it remains in use in all multi-dose vaccines and flu shots (though many single use vaccines without thimerosal are available).[8] Researchers have argued that risk assessments based on methylmercury were overly conservative in light of observations that ethylmercury is eliminated from the body and the brain significantly faster than methylmercury.[1] Moreover, the same researchers have argued that inorganic mercury metabolized from ethylmercury, despite its much longer half-life in the brain, is much less toxic than the inorganic mercury produced from mercury vapor, for reasons not yet understood.[1]
See also
References and notes
- ↑ 1.0 1.1 1.2 1.3 1.4 Clarkson, Thomas W.; Magos, Laszlo (September 2006). "The toxicology of mercury and its chemical compounds". Critical Reviews in Toxicology 36 (8): 609–62. doi:10.1080/10408440600845619. PMID 16973445.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Elschenbroich, Christoph (2016). "Main-Group Organometallics [§6.2.3 Organomercury Compounds"]. Organometallics (3rd ed.). New York, NY: John Wiley & Sons. pp. 78–86. ISBN 978-3-527-80514-3. https://books.google.com/books?isbn=3527805141. Retrieved 13 February 2017.
- ↑ Counter, S.Allen; Buchanan, Leo H. (2004). "Mercury exposure in children: A review". Toxicology and Applied Pharmacology 198 (2): 209–230. doi:10.1016/j.taap.2003.11.032. PMID 15236954.
- ↑ "Mechanisms of mercury disposition in the body". American Journal of Industrial Medicine 50 (10): 757–64. October 2007. doi:10.1002/ajim.20476. PMID 17477364.
- ↑ "Mercury exposure and public health". Pediatric Clinics of North America 54 (2): 237–69, viii. April 2007. doi:10.1016/j.pcl.2007.02.005. PMID 17448359.[verification needed]
- ↑ "Weekly Epidemiological Record, vol. 87, 30 (pp 277–288)". 2012-07-27. https://www.who.int/wer/2012/wer8730/en/.
- ↑ Research, Center for Biologics Evaluation and (2019-04-05). "Thimerosal and Vaccines" (in en). FDA. http://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/thimerosal-and-vaccines.
Further reading
- "Carbohydrate assimilation by saccharolytic clostridia". Environmental Health Perspectives 143 (3): 245–50. 2005. doi:10.1289/ehp.113-a543.
- DHHS ATSDR, US (March 2013). "Addendum to the Toxicological Profile for Mercury (Alkyl and Dialkyl Compounds)". CDC.gov. https://www.atsdr.cdc.gov/toxprofiles/mercury_organic_addendum.pdf.
- EPA, OA, US (9 September 2015). "Thimerosal in Vaccines". EPA.gov. https://www.epa.gov/mercury/thimerosal-vaccines.
- "Thimerosal changes protein conformation and increase the rate of fibrillation in physiological conditions: Spectroscopic studies using bovine serum albumin (BSA)". International Journal of Biological Macromolecules 113: 1032–1040. February 2018. doi:10.1016/j.ijbiomac.2018.02.116. PMID 29476861.
External links
- EPA Organic Mercury TEACH Chemical Summary, 2007.
- EPA Chemistry Dashboard, Ethyl Mercury Ion, 2017.
- ATSDR Toxicological Profile for Mercury, search "Organic Mercury".
 |