Chemistry:Mercury(I) sulfate
| |
| |
| Names | |
|---|---|
| IUPAC name
Mercury(I) sulfate
| |
| Other names
Mercurous sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Hg2SO4 | |
| Molar mass | 497.24 g/mol |
| Appearance | whitish-yellow crystals |
| Density | 7.56 g/cm3 |
| 0.051 g/100 mL (25 °C) 0.09 g/100 mL (100 °C) | |
Solubility product (Ksp)
|
6.5×10−7[1] |
| Solubility | soluble in dilute nitric acid, Insoluble in water, Soluble in hot sulfuric acid. |
| −123.0·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| Thermochemistry | |
Heat capacity (C)
|
132 J·mol−1·K−1[2] |
Std molar
entropy (S |
200.7 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
-743.1 kJ·mol−1 |
| Related compounds | |
Other anions
|
Mercury(I) fluoride Mercury(I) chloride Mercury(I) bromide Mercury(I) iodide |
Other cations
|
Mercury(II) sulfate Cadmium sulfate Thallium(I) sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mercury(I) sulfate, commonly called mercurous sulphate (United Kingdom ) or mercurous sulfate (United States ) is the chemical compound Hg2SO4.[3] Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder.[4] It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin.
Structure
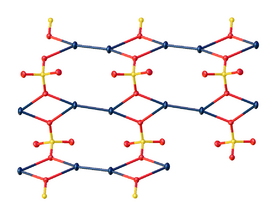
In the crystal, mercurous sulfate is made up of Hg22+ center with an Hg-Hg distance of about 2.50 Å. The SO42− anions form both long and short Hg-O bonds ranging from 2.23 to 2.93 Å.[5]
Focusing on the shorter Hg-O bonds, the Hg – Hg – O bond angle is 165°±1°.[6][7]
Preparation
One way to prepare mercury(I) sulfate is to mix the acidic solution of mercury(I) nitrate with 1 to 6 sulfuric acid solution:,[8][9]
- Hg2(NO3)2 + H2SO4 → Hg2SO4 + 2 HNO3
It can also be prepared by reacting an excess of mercury with concentrated sulfuric acid:[8]
- 2 Hg + 2 H2SO4 → Hg2SO4 + 2 H2O + SO2
Use in electrochemical cells
Mercury(I) sulfate is often used in electrochemical cells.[10][11][12] It was first introduced in electrochemical cells by Latimer Clark in 1872,[13] It was then alternatively[clarification needed] used in Weston cells made by George Augustus Hulett in 1911.[13] It has been found to be a good electrode at high temperatures above 100 °C along with silver sulfate.[14]
Mercury(I) sulfate has been found to decompose at high temperatures. The decomposition process is endothermic, and it occurs between 335 °C and 500 °C.
Mercury(I) sulfate has unique properties that make the standard cells possible. It has a rather low solubility (about one gram per liter); diffusion from the cathode system is not excessive; and it is sufficient to give a large potential at a mercury electrode.[15]
References
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–189. ISBN 978-1-138-56163-2.
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 5–19, ISBN 0-8493-0594-2
- ↑ Intermediate Inorganic Chemistry by J. W. Mellor, published by Longmans, Green and Company, London, 1941, page 388
- ↑ "Mercurous Sulfate | 7783-36-0". http://www.chemicalbook.com/ChemicalProductProperty_EN_CB0259783.htm.
- ↑ Matthias Weil; Michael Puchberger; Enrique J. Baran (2004). "Preparation and Characterization of Dimercury(I)Monofluorophosphate(V), Hg2PO3F: Crystal Structure, Thermal Behavior, Vibrational Spectra, and Solid-State 31P and 19F NMR Spectra". Inorg. Chem. 43 (26): 8330–8335. doi:10.1021/ic048741e. PMID 15606179.
- ↑ Dorm, E. (1969). "Structural Studies on Mercury(I) Compounds. VI. Crystal Structure of Mercury(I) Sulfate and Selenate". Acta Chemica Scandinavica 23: 1607–15. doi:10.3891/acta.chem.scand.23-1607.
- ↑ Weil, Matthias (2014). "Crystal structure of Hg2SO4– a redetermination". Acta Crystallographica Section E 70 (9): i44. doi:10.1107/S1600536814011155. PMID 25309168.
- ↑ 8.0 8.1 Google Books result, accessed 11 December 2010
- ↑ Mercurous Sulphate, cadmium sulphate, and the cadmium cell. by Hulett G. A. The physical review.1907. p.19.
- ↑ "Influence of Microstucture on the Charge Storage Properties of Chemically Synthesized Manganese Dioxide" by Mathieu Toupin, Thiery Brousse, and Daniel Belanger. Chem. Mater. 2002, 14, 3945–3952
- ↑ "Electromotive Force Studies of Cell, CdxHgy | CdSO4,(m) I Hg2SO4, Hg, in Dioxane-Water Media" by Somesh Chakrabarti and Sukumar Aditya. Journal of Chemical and Engineering Data, Vol.17, No. 1, 1972
- ↑ "Characterization of Lithium Sulfate as an Unsymmetrical-Valence Salt Bridge for the Minimization of Liquid Junction Potentials in Aqueous – Organic Solvent Mixtures" by Cristiana L. Faverio, Patrizia R. Mussini, and Torquato Mussini. Anal. Chem. 1998, 70, 2589–2595
- ↑ 13.0 13.1 "George Augustus Hulett: from Liquid Crystals to Standard Cell" by John T. Stock. Bull. Hist. Chem. Volume 25, Number 2, 2000, p.91-98
- ↑ Lietzke, M. H.; Stoughton, R. W. (November 1953). "The Behavior of the Silver—Silver Sulfate and the Mercury—Mercurous Sulfate Electrodes at High Temperatures 1". Journal of the American Chemical Society 75 (21): 5226–5227. doi:10.1021/ja01117a024.(Subscription content?)
- ↑ "Sulphates of Mercury and Standard Cells." by Elliott, R. B. and Hulett, G. A. The Journal of Physical Chemistry 36.7 (1932): 2083–2086.
 |

