Chemistry:Mercury(II) fulminate
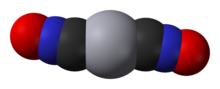
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Mercury(II) fulminate
| |
| Systematic IUPAC name
Dioxycyanomercury | |
| Other names
Fulminated Mercury
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2N2O2Hg | |
| Molar mass | 284.624 g/mol |
| Appearance | Grey, Pale Brown, or White Crystalline solid |
| Density | 4.42 g/cm3 |
| Melting point | 160 °C (320 °F; 433 K) |
| Boiling point | 356.6 °C (673.9 °F; 629.8 K) |
| slightly soluble | |
| Solubility | soluble in ethanol, ammonia |
| Explosive data | |
| Shock sensitivity | High |
| Friction sensitivity | High |
| Detonation velocity | 4250 m/s |
| Hazards | |
| Main hazards | Highly Toxic, Shock Sensitive Explosive |
| NFPA 704 (fire diamond) | |
| 170 °C (338 °F; 443 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mercury(II) fulminate, or Hg(CNO)2, is a primary explosive. It is highly sensitive to friction, heat and shock and is mainly used as a trigger for other explosives in percussion caps and detonators. Mercury(II) cyanate, though its chemical formula is identical, has a different atomic arrangement; the cyanate and fulminate anions are isomers.
First used as a priming composition in small copper caps beginning in the 1820s, mercury fulminate quickly replaced flints as a means to ignite black powder charges in muzzle-loading firearms. Later, during the late 19th century and most of the 20th century, mercury fulminate became widely used in primers for self-contained rifle and pistol ammunition; it was the only practical detonator for firing projectiles until the early 20th century.[1] Mercury fulminate has the distinct advantage over potassium chlorate of being non-corrosive, but it is known to weaken with time, by decomposing into its constituent elements. The reduced mercury which results forms amalgams with cartridge brass, weakening it, as well. Today, mercury fulminate has been replaced in primers by more efficient chemical substances. These are non-corrosive, less toxic, and more stable over time; they include lead azide, lead styphnate, and tetrazene derivatives. In addition, none of these compounds require mercury for manufacture, supplies of which can be unreliable in wartime.
Preparation
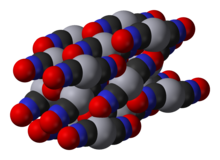
Mercury(II) fulminate is prepared by dissolving mercury in nitric acid and adding ethanol to the solution. It was first prepared by Edward Charles Howard in 1800.[2][1] The crystal structure of this compound was determined only in 2007.[3]
Silver fulminate can be prepared in a similar way, but this salt is even more unstable than mercury fulminate; it can explode even under water and is impossible to accumulate in large amounts because it detonates under its own weight.[4]
Decomposition
The thermal decomposition of mercury(II) fulminate can begin at temperatures as low as 100 °C, though it proceeds at a much higher rate with increasing temperature.[5]
A possible reaction for the decomposition of mercury(II) fulminate yields carbon dioxide gas, nitrogen gas, and a combination of relatively stable mercury salts.
- 4 Hg(CNO)2 → 2 CO2 + N2 + HgO + 3 Hg(OCN)CN
- Hg(CNO)2 → 2 CO + N2 + Hg
- Hg(CNO)2 → :Hg(OCN)2 (cyanate or / and isocyanate)
- 2 Hg(CNO)2 → 2 CO2 + N2 + Hg + Hg(CN)2 (mercury(II) cyanide)
In popular culture
In the 1955 film Mister Roberts, Ensign Pulver (Jack Lemmon) acquires fulminated mercury. Intending to blow up his unpopular captain's bunk as a prank, he accidentally destroys the ship's laundry instead.
In the episode "Crazy Handful of Nothin'" of the television series Breaking Bad, Walter White uses fulminated mercury "with a little tweak of chemistry" to create an explosion in meth kingpin Tuco Salamanca's office as an intimidation tactic. The depiction of the substance and its effects in the show is questionable in accuracy; the crystals themselves, as shown, would be too large to survive handling without exploding, and when Walt throws a crystal at the ground the resulting explosion ruins the office but leaves the occupants unharmed;[6] however, it is not explained what White means by a tweak of chemistry or how that may have affected the crystal's explosive properties.
See also
References
- ↑ 1.0 1.1 Wisniak, Jaime (2012). "Edward Charles Howard. Explosives, meteorites, and sugar" (in en). Educación Química (Universidad Nacional Autonoma de Mexico) 23 (2): 230–239. doi:10.1016/s0187-893x(17)30114-3. ISSN 0187-893X.
- ↑ Edward Howard (1800). "On a New Fulminating Mercury". Philosophical Transactions of the Royal Society of London 90 (1): 204–238. doi:10.1098/rstl.1800.0012.
- ↑ W. Beck; J. Evers; M. Göbel; G. Oehlinger; T. M. Klapötke (2007). "The Crystal and Molecular Structure of Mercury Fulminate (Knallquecksilber)". Zeitschrift für anorganische und allgemeine Chemie 633 (9): 1417–1422. doi:10.1002/zaac.200700176.
- ↑ "The Sciences - Fulminating Substances". 11 June 1853. https://www.scientificamerican.com/article/fulminating-substances-1853-06-11/.
- ↑ W. E. Garner; H. R. Hailes (1933). "Thermal decomposition and detonation of mercury fulminate". Proceedings of the Royal Society of London 139 (1–3): 1–40. doi:10.1098/rspa.1933.0040. Bibcode: 1933CP....334..128S.
- ↑ Hare2012-11-01T10:38:00+00:00, Jonathan. "Breaking Bad IV – can a little crystal blow up a room?" (in en). https://edu.rsc.org/analysis/breaking-bad-iv-can-a-little-crystal-blow-up-a-room/3007376.article.
External links
- National Pollutant Inventory - Mercury and compounds Fact Sheet
- "300 years after discovery, structure of mercury fulminate finally determined". physorg.com. 24 August 2007. http://www.physorg.com/news107176552.html.
 |

