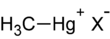Chemistry:Methylmercury
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| CH3Hg | |||
| Molar mass | 215.63 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula [CH3Hg]+. It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environmental toxicant with a 50-day half-life.[1]
Structure and chemistry
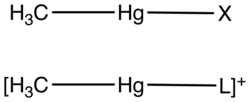
"Methylmercury" is a shorthand for the hypothetical "methylmercury cation", sometimes written methylmercury(1+) cation or methylmercury(II) cation. This functional group is composed of a methyl group bonded to an atom of mercury. Its chemical formula is CH
3Hg+
(sometimes written as MeHg+
).The Methylmercury compound has an overall charge of +1, with Hg in the +2 oxidation state. Methylmercury exists as a substituent in many complexes of the type [MeHgL]+
(L = Lewis base) and MeHgX (X = anion).[2]
As a positively charged ion, it readily combines with anions such as chloride (Cl−
), hydroxide (OH−
) and nitrate (NO−
3). It has particular affinity for sulfur-containing anions, particularly thiols (RS−
). Thiols are generated when the amino acid cysteine and the peptide glutathione form strong complexes with methylmercury:[3]
- [MeHg]+
+ RSH → MeHg–SR + H+
Sources
Environmental sources

Methylmercury is formed from inorganic mercury by the action of microbes that live in aquatic systems including lakes, rivers, wetlands, sediments, soils and the open ocean.[5] This methylmercury production has been primarily attributed to anaerobic bacteria in the sediment.[6] Significant concentrations of methylmercury in ocean water columns[7] are strongly associated with nutrients and organic matter remineralization, which indicate that remineralization may contribute to methylmercury production.[8] Direct measurements of methylmercury production using stable mercury isotopes have also been observed in marine waters,[9][10] but the microbes involved are still unknown. Increased methylmercury concentrations in water and fish have been detected after flooding of soils associated with reservoir creation (e.g. for hydroelectric power generation) and in thermokarst wetlands that form after permafrost thaw.[9][11][12]
There are various sources of inorganic mercury that may indirectly contribute to the production of methylmercury from microbes in the environment. Natural sources of mercury released to the atmosphere include volcanoes, forest fires, volatilization from the ocean[13] and weathering of mercury-bearing rocks.[14] Anthropogenic sources of mercury include the burning of wastes containing inorganic mercury and from the burning of fossil fuels, particularly coal. Although inorganic mercury is only a trace constituent of such fuels, their large scale combustion in utility and commercial/industrial boilers in the United States alone results in release of some 80.2 tons (73 metric tons) of elemental mercury to the atmosphere each year, out of total anthropogenic mercury emissions in the United States of 158 tons (144 metric tons)/year.[15]
In the past, methylmercury was produced directly and indirectly as part of several industrial processes such as the manufacture of acetaldehyde. However, currently there are few direct anthropogenic sources of methylmercury pollution in the United States.[15]
Whole-lake ecosystem experiments at IISD-ELA in Ontario, Canada, showed that mercury falling directly on a lake had the fastest impacts on aquatic ecosystems as opposed to mercury falling on the surrounding land.[16] This inorganic mercury is converted to methylmercury by bacteria. Different stable isotopes of mercury were added to lakes, wetlands, and uplands, simulating rain, and then mercury concentrations in fish were analyzed to find their source.[17] The mercury applied to lakes was found in young-of-the-year yellow perch within two months, whereas the mercury applied to wetlands and uplands had a slower but longer influx.[16][17]
Acute methylmercury poisoning can occur either directly from the release of methylmercury into the environment or indirectly from the release of inorganic mercury that is subsequently methylated in the environment. For example, methylmercury poisoning occurred at Grassy Narrows in Ontario, Canada (see Ontario Minamata disease), as a result of mercury released from the mercury-cell Chloralkali process, which uses liquid mercury as an electrode in a process that entails electrolytic decomposition of brine, followed by mercury methylation in the aquatic environment. An acute methylmercury poisoning tragedy occurred also in Minamata, Japan, following release of methylmercury into Minamata Bay and its tributaries (see Minamata disease). In the Ontario case, inorganic mercury discharged into the environment was methylated in the environment; whereas, in Minamata, Japan, there was direct industrial discharge of methylmercury.
Dietary sources
Because methylmercury is formed in aquatic systems, and because it is not readily eliminated from organisms, it is biomagnified in aquatic food chains from bacteria, to plankton, through macroinvertebrates, to herbivorous fish and to piscivorous (fish-eating) fish.[18][19] At each step in the food chain, the concentration of methylmercury in the organism increases. The concentration of methylmercury in the top-level aquatic predators can reach a level a million times higher than the level in the water.[18][19] This is because methylmercury has a half-life of about 72 days in aquatic organisms resulting in its bioaccumulation within these food chains. Organisms, including humans,[20] fish-eating birds, and fish-eating mammals such as otters and cetaceans (i.e. whales and dolphins) that consume fish from the top of the aquatic food chain receive the methylmercury that has accumulated through this process, plus the toxins in their habitat.[18][19] Fish and other aquatic species are the main source of human methylmercury exposure.[18]
The concentration of mercury in any given fish depends on the species of fish, the age and size of the fish and the type of water body in which it is found.[18] In general, fish-eating fish such as shark, swordfish, marlin, larger species of tuna, walleye, largemouth bass, and northern pike, have higher levels of methylmercury than herbivorous fish or smaller fish such as tilapia and herring.[21][22] Within a given species of fish, older and larger fish have higher levels of methylmercury than smaller fish. Fish that develop in water bodies that are more acidic also tend to have higher levels of methylmercury.[18]
Biological impact
Human health effects
Ingested methylmercury is readily and completely absorbed by the gastrointestinal tract. It is mostly found complexed with free cysteine and with proteins and peptides containing that amino acid. The methylmercuric-cysteinyl complex is recognized by amino acids transporting proteins in the body as methionine, another essential amino acid.[23] Because of this mimicry, it is transported freely throughout the body including across the blood–brain barrier and across the placenta, where it is absorbed by the developing fetus. Also for this reason as well as its strong binding to proteins, methylmercury is not readily eliminated. Methylmercury has a half-life in human blood of about 50 days.[24]
Several studies indicate that methylmercury is linked to subtle developmental deficits in children exposed in utero such as loss of IQ points, and decreased performance in tests of language skills, memory function and attention deficits.[25] Methylmercury exposure in adults has also been linked to increased risk of cardiovascular disease including heart attack.[26][27][28] Some evidence also suggests that methylmercury can cause autoimmune effects in sensitive individuals.[29] Despite some concerns about the relationship between methylmercury exposure and autism, there are few data that support such a link.[30] Although there is no doubt that methylmercury is toxic in several respects, including through exposure of the developing fetus, there is still some controversy as to the levels of methylmercury in the diet that can result in adverse effects. Recent evidence suggests that the developmental and cardiovascular toxicity of methylmercury may be mitigated by co-exposures to omega-3 fatty acids and perhaps selenium, both found in fish and elsewhere.[27][31][32][33][34]
There have been several episodes in which large numbers of people were severely poisoned by food contaminated with high levels of methylmercury, notably the dumping of industrial waste that resulted in the pollution and subsequent mass poisoning in Minamata and Niigata, Japan [35] and the situation in Iraq in the 1960s and 1970s in which wheat treated with methylmercury as a preservative and intended as seed grain was fed to animals and directly consumed by people (see Basra poison grain disaster). These episodes resulted in neurological symptoms including paresthesias, loss of physical coordination, difficulty in speech, narrowing of the visual field, hearing impairment, blindness, and death. Children who had been exposed in utero through their mothers' ingestion were also affected with a range of symptoms including motor difficulties, sensory problems and intellectual disability.
At present, exposures of this magnitude are rarely seen and are confined to isolated incidents. Accordingly, concern over methylmercury pollution is currently focused on more subtle effects that may be linked to levels of exposure presently seen in populations with high to moderate levels of dietary fish consumption. These effects are not necessarily identifiable on an individual level or may not be uniquely recognizable as due to methylmercury. However, such effects may be detected by comparing populations with different levels of exposure. There are isolated reports of various clinical health effects in individuals who consume large amounts of fish;[36] however, the specific health effects and exposure patterns have not been verified with larger, controlled studies.
Many governmental agencies, the most notable ones being the United States Environmental Protection Agency (EPA), the United States Food and Drug Administration (FDA), Health Canada, and the European Union Health and Consumer Protection Directorate-General, as well as the World Health Organization (WHO) and the United Nations Food and Agriculture Organization (FAO), have issued guidance for fish consumers that is designed to limit methylmercury exposure from fish consumption. At present, most of this guidance is based on protection of the developing fetus; future guidance, however, may also address cardiovascular risk. In general, fish consumption advice attempts to convey the message that fish is a good source of nutrition and has significant health benefits, but that consumers, in particular pregnant women, women of child-bearing age, nursing mothers, and young children, should avoid fish with high levels of methylmercury, limit their intake of fish with moderate levels of methylmercury, and consume fish with low levels of methylmercury no more than twice a week.[37][38]
Effects on fish and wildlife
File:NATIONAL WATER QUALITY LABORATORY, 30 DAY GROWTH OF JORDANELLA. THE BOTTLES, LEFT TO RIGHT, CONTAIN, 1-THE CONTROL... - NARA - 551591.tif In recent years, there has been increasing recognition that methylmercury affects fish and wildlife health, both in acutely polluted ecosystems and ecosystems with modest methylmercury levels. Two reviews[18][39] document numerous studies of diminished reproductive success of fish, fish-eating birds, and mammals due to methylmercury contamination in aquatic ecosystems.
In public policy
Reported methylmercury levels in fish, along with fish consumption advisories, have the potential to disrupt people's eating habits, fishing traditions, and the livelihoods of the people involved in the capture, distribution, and preparation of fish as a foodstuff for humans.[40] Furthermore, proposed limits on mercury emissions have the potential to add costly pollution controls on coal-fired utility boilers. Nevertheless, substantial benefits can be achieved globally by introducing mercury emission reduction measures because they reduce human and wildlife exposure to methylmercury.[41]
About 30% of the distributed mercury depositional input is from current anthropogenic sources, and 70% is from natural sources. The natural sources category includes re-emission of mercury previously deposited from anthropogenic sources.[42] According to one study, based on modeled concentrations, pre-Anthropocene tissue-bound levels in freshwater fish may not have differed markedly from current levels.[43] However, based on a comprehensive set of global measurements, the ocean contains about 60,000 to 80,000 tons of mercury from pollution, and mercury levels in the upper ocean have tripled since the beginning of the industrial revolution. Higher mercury levels in shallower ocean waters could increase the amount of the toxicant accumulating in food fish, exposing people to a greater risk of mercury poisoning.[44]
See also
- Canadian Reference Materials include some with methylmercury, e.g. DORM
- Dimethylmercury, mercury with a second methyl group
- Ethylmercury, a related cation
- Mercury poisoning
- Mercury regulation in the United States
References
- ↑ Halliday, Tim; Davey, Basiro (2007). Water and health in an overcrowded world. Oxford: Oxford University Press. pp. 79, 80, 95. ISBN 9780199237302.
- ↑ Canty, Allan J.; Chaichit, Narongsak; Gatehouse, Bryan M.; George, Edwin E.; Hayhurst, Glen (1981). "Coordination chemistry of methylmercury(II). Synthesis, hydrogen-1 NMR, and crystallographic studies of cationic complexes of Me Hg(II) with ambidentate and polydentate ligands containing pyridyl and N-substituted imidazolyl donors and involving unusual coordination geometries". Inorganic Chemistry 20 (8): 2414–2422. doi:10.1021/ic50222a011.
- ↑ Nolan, Elizabeth M.; Lippard, Stephen J. (2008). "Tools and Tactics for the Optical Detection of Mercuric Ion". Chemical Reviews 108 (9): 3443–3480. doi:10.1021/cr068000q. PMID 18652512.
- ↑ Taylor, Nicholas J.; Wong, Yau S.; Chieh, Peter C.; Carty, Arthur J. (1975). "Syntheses, X-ray crystal structure, and vibrational spectra of L-cysteinato(methyl)mercury(II) monohydrate". Journal of the Chemical Society, Dalton Transactions (5): 438. doi:10.1039/DT9750000438.
- ↑ Ullrich, Susanne; Tanton, Trevor; Abdrashitova, Svetlana (2001). "Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation". Critical Reviews in Environmental Science and Technology 31 (3): 241–293. doi:10.1080/20016491089226.
- ↑ Compeau, G.C.; Bartha, R. (1985-08-01). "Sulfate-Reducing Bacteria: Principal Methylators of Mercury in Anoxic Estuarine Sediment". Applied and Environmental Microbiology 50 (2): 498–502. doi:10.1128/AEM.50.2.498-502.1985. ISSN 0099-2240. PMID 16346866. Bibcode: 1985ApEnM..50..498C.
- ↑ Mason, R.P.; Fitzgerald, W.F. (1990-10-04). "Alkylmercury species in the equatorial Pacific". Nature 347 (6292): 457–459. doi:10.1038/347457a0. Bibcode: 1990Natur.347..457M.
- ↑ Sunderland, Elsie M.; Krabbenhoft, David P.; Moreau, John W.; Strode, Sarah A.; Landing, William M. (2009-06-01). "Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models". Global Biogeochemical Cycles 23 (2): GB2010. doi:10.1029/2008GB003425. ISSN 1944-9224. Bibcode: 2009GBioC..23.2010S.
- ↑ 9.0 9.1 Schartup, Amina T.; Balcom, Prentiss H.; Soerensen, Anne L.; Gosnell, Kathleen J.; Calder, Ryan S.D.; Mason, Robert P.; Sunderland, Elsie M. (2015-09-22). "Freshwater discharges drive high levels of methylmercury in Arctic marine biota". Proceedings of the National Academy of Sciences 112 (38): 11789–11794. doi:10.1073/pnas.1505541112. ISSN 0027-8424. PMID 26351688. Bibcode: 2015PNAS..11211789S.
- ↑ Lehnherr, Igor; St.Louis, Vincent L.; Hintelmann, Holger; Kirk, Jane L. (2011). "Methylation of inorganic mercury in polar marine waters". Nature Geoscience 4 (5): 298–302. doi:10.1038/ngeo1134. Bibcode: 2011NatGe...4..298L.
- ↑ St.Louis, Vincent L.; Rudd, John W.M.; Kelly, Carol A.; Bodaly, R.A. (Drew); Paterson, Michael J.; Beaty, Kenneth G.; Hesslein, Raymond H.; Heyes, Andrew et al. (2004-03-01). "The Rise and Fall of Mercury Methylation in an Experimental Reservoir". Environmental Science & Technology 38 (5): 1348–1358. doi:10.1021/es034424f. ISSN 0013-936X. PMID 15046335.
- ↑ Tarbier, Brittany; Hugelius, Gustaf; Kristina Sannel, Anna Britta; Baptista-Salazar, Carluvy; Jonsson, Sofi (2021-04-26). "Permafrost Thaw Increases Methylmercury Formation in Subarctic Fennoscandia". Environmental Science & Technology 55 (10): 6710–6717. doi:10.1021/acs.est.0c04108. ISSN 0013-936X. PMID 33902281. Bibcode: 2021EnST...55.6710T.
- ↑ "Mercury in the Environment". U.S. Geological Survey. http://www.usgs.gov/themes/factsheet/146-00/.
- ↑ Tewalt, S. J.; Bragg, L. J.; Finkelman, R. B., 2005, Mercury in U.S. coal -- Abundance, distribution, and modes of occurrence, U.S. Geological Survey Fact Sheet 095-01. Access-date=January 12, 2006.
- ↑ 15.0 15.1 U. S. Environmental Protection Agency, 1997, "Mercury study report to congress, Volume II: An inventory of anthropogenic mercury emissions in the United States" , table ES-3, sum of Utility boilers and Commercial/industrial boilers. Report: EPA-452/R-97-004.
- ↑ 16.0 16.1 "Mercury: What it does to humans and what humans need to do about it". 2017-09-23. https://www.iisd.org/ela/blog/commentary/mercury-humans-humans-need/.
- ↑ 17.0 17.1 Grieb, Thomas M.; Fisher, Nicholas S.; Karimi, Roxanne; Levin, Leonard (2019-10-03). "An assessment of temporal trends in mercury concentrations in fish". Ecotoxicology 29 (10): 1739–1749. doi:10.1007/s10646-019-02112-3. ISSN 1573-3017. PMID 31583510.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 reviewed in Wiener, J.G., Krabbenhoft, D.P., Heinz, G.H., and Scheuhammer, A.M., 2003, "Ecotoxicology of mercury", Chapter 16 in Hoffman, D.J., B.A. Rattner, G.A. Burton, Jr., and J. Cairns, Jr., eds., Handbook of Ecotoxicology, 2nd edition.: Boca Raton, FL: CRC Press, p. 409–463.
- ↑ 19.0 19.1 19.2 Lavoie, Raphael A.; Jardine, Timothy D.; Chumchal, Matthew M.; Kidd, Karen A.; Campbell, Linda M. (2013-11-13). "Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis". Environmental Science & Technology 47 (23): 13385–13394. doi:10.1021/es403103t. ISSN 0013-936X. PMID 24151937. Bibcode: 2013EnST...4713385L.
- ↑ Burros, Marian (2008-01-23). "High Mercury Levels Are Found in Tuna Sushi". The New York Times. https://www.nytimes.com/2008/01/23/dining/23sushi.html.
- ↑ Mercury Levels in Commercial Fish and Shellfish Accessed March 25, 2009.
- ↑ What You Need to Know about Mercury in Fish and Shellfish Accessed March 25, 2009.
- ↑ Kerper, L.; Ballatori, N.; Clarkson, T.W. (May 1992). "Methylmercury transport across the blood–brain barrier by an amino acid carrier". American Journal of Physiology 262 (5 Pt 2): R761–765. doi:10.1152/ajpregu.1992.262.5.R761. PMID 1590471.
- ↑ Carrier, G; Bouchard, M; Brunet, RC; Caza, M (2001). "A Toxicokinetic Model for Predicting the Tissue Distribution and Elimination of Organic and Inorganic Mercury Following Exposure to Methyl Mercury in Animals and Humans. II. Application and Validation of the Model in Humans". Toxicology and Applied Pharmacology 171 (1): 50–60. doi:10.1006/taap.2000.9113. PMID 11181111.
- ↑ Rice, DC; Schoeny, R; Mahaffey, K (2003). "Methods and rationale for derivation of a reference dose for methylmercury by the U.S. EPA". Risk Analysis 23 (1): 107–115. doi:10.1111/1539-6924.00294. PMID 12635727. https://zenodo.org/record/1230585.
- ↑ Salonen, J. T.; Seppänen, K.; Nyyssönen, K.; Korpela, H.; Kauhanen, J.; Kantola, M.; Tuomilehto, J.; Esterbauer, H. et al. (1995). "Intake of Mercury from Fish, Lipid Peroxidation, and the Risk of Myocardial Infarction and Coronary, Cardiovascular, and Any Death in Eastern Finnish Men". Circulation 91 (3): 645–655. doi:10.1161/01.CIR.91.3.645. PMID 7828289.
- ↑ 27.0 27.1 Guallar, E; Sanz-Gallardo, MI; Van't Veer, P; Bode, P; Aro, A; Gómez-Aracena, J; Kark, JD; Riemersma, RA et al. (2002). "Mercury, fish oils, and the risk of myocardial infarction". The New England Journal of Medicine 347 (22): 1747–1754. doi:10.1056/NEJMoa020157. PMID 12456850.
- ↑ Choi, A.L., Weihe, P., Budtz-Jørgensen, E., Jørgensen, P.J., Salonen, J.T., Tuomainen, T.-P., Murata, K., Nielsen, H.P., Petersen, M.S., Askham, J., and Grandjean, P., 2009, Methylmercury Exposure and Adverse Cardiovascular Effects in Faroese Whaling Men: Environmental Health Perspectives, v. 117, no. 3, p. 367–372.
- ↑ Hultman, P; Hansson-Georgiadis, H (1999). "Methyl mercury–induced autoimmunity in mice". Toxicology and Applied Pharmacology 154 (3): 203–211. doi:10.1006/taap.1998.8576. PMID 9931279.
- ↑ https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/B/excipient-table-2.pdf [bare URL PDF]
- ↑ Choi, AL; Cordier, S; Weihe, P; Grandjean, P (2008). "Negative confounding in the evaluation of toxicity: The case of methylmercury in fish and seafood". Critical Reviews in Toxicology 38 (10): 877–893. doi:10.1080/10408440802273164. PMID 19012089. Review. Erratum in: "Erratum". Critical Reviews in Toxicology 39: 95. 2009. doi:10.1080/10408440802661707.
- ↑ Strain, JJ; Davidson, PW; Bonham, MP; Duffy, EM; Stokes-Riner, A; Thurston, SW; Wallace, JM; Robson, PJ et al. (2008). "Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study". Neurotoxicology 29 (5): 776–82. doi:10.1016/j.neuro.2008.06.002. PMID 18590765.
- ↑ Khan, MA; Wang, F (2009). "Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism". Environmental Toxicology and Chemistry 28 (8): 1567–77. doi:10.1897/08-375.1. PMID 19374471. Review.
- ↑ Heath, JC; Banna, KM; Reed, MN; Pesek, EF; Cole, N; Li, J; Newland, MC (2010). "Dietary selenium protects against selected signs of aging and methylmercury exposure". Neurotoxicology 31 (2): 169–79. doi:10.1016/j.neuro.2010.01.003. PMID 20079371.
- ↑ Myers, G.J.; Davidson, P.W.; Weiss, B. (2004). "Methyl mercury exposure and poisoning at Niigata, Japan". SMDJ Seychelles Medical and Dental Journal 7 (Special Issue): 132–133. http://www.seychelles.net/smdj/SECVA.pdf. Retrieved January 12, 2006.
- ↑ For example: Hightower, JM; Moore, D (2003). "Mercury levels in high-end consumers of fish". Environmental Health Perspectives 111 (4): 604–8. doi:10.1289/ehp.5837. PMID 12676623.
- ↑ Information on characteristic levels of methylmercury by species can be found at "FDA - Mercury Levels in Commercial Fish and Shellfish". http://www.cfsan.fda.gov/~frf/sea-mehg.html.
- ↑ A wallet-card guide for consumers can be found at http://www.nrdc.org/health/effects/mercury/protect.asp
- ↑ Scheuhammer, Anton M.; Meyer, Michael W.; Sandheinrich, Mark B.; Murray, Michael W. (2007). "Effects of Environmental Methylmercury on the Health of Wild Birds, Mammals, and Fish". Ambio: A Journal of the Human Environment 36 (1): 12–19. doi:10.1579/0044-7447(2007)36[12:EOEMOT2.0.CO;2]. ISSN 0044-7447. PMID 17408187.
- ↑ Wheatley, B; Wheatley, M (2000). "Methylmercury and the health of indigenous peoples: a risk management challenge for physical and social sciences and for public health policy". The Science of the Total Environment 259 (1–3): 23–29. doi:10.1016/S0048-9697(00)00546-5. PMID 11032132. Bibcode: 2000ScTEn.259...23W.
- ↑ Jozef M. Pacyna, Kyrre Sundseth, Elisabeth G. Pacyna, Wojciech Jozewicz, John Munthe, Mohammed Belhaj & Stefan Aström (2010), "An Assessment of Costs and Benefits Associated with Mercury Emission Reductions from Major Anthropogenic Sources", Journal of the Air & Waste Management Association, 60:3, 302–315, DOI: 10.3155/1047-3289.60.3.302
- ↑ Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B. et al. (2010). "Global Mercury Emissions to the Atmosphere from Anthropogenic and Natural Sources". Atmospheric Chemistry and Physics 10 (13): 5951–5964. doi:10.5194/acp-10-5951-2010. Bibcode: 2010ACP....10.5951P.
- ↑ Hope, Bruce K.; Louch, Jeff (2013). "Pre-Anthropocene Mercury Residues in North American Freshwater Fish". Integrated Environmental Assessment and Management 10 (2): 299–308. doi:10.1002/ieam.1500. PMID 24458807.
- ↑ Carl H. Lamborg, Chad R. Hammerschmidt, Katlin L. Bowman, Gretchen J. Swarr, Kathleen M. Munson, Daniel C. Ohnemus, Phoebe J. Lam, Lars-Eric Heimbürger, Micha J. A. Rijkenberg & Mak A. Saito (2014) A global ocean inventory of anthropogenic mercury based on water column measurements, Nature, 512, 65–68, doi:10.1038/nature13563
External links
- ATSDR - ToxFAQs: Mercury
- ATSDR - Public Health Statement: Mercury
- ATSDR - ALERT! Patterns of Metallic Mercury Exposure, 6/26/97
- ATSDR - MMG: Mercury
- ATSDR - Toxicological Profile: Mercury
- National Pollutant Inventory - Mercury and compounds Fact Sheet
- Methylmercury-in-fish exposure calculator provided by GotMercury.Org, which uses FDA mercury data with the EPA's calculated safe exposure levels.
- Methylmercury Contamination in Fish and Shellfish
- nytimes.com, Tuna Fish Stories: The Candidates Spin the Sushi
- U.S. Environmental Protection Agency's mercury site
- U.S. Geological Survey's mercury site
- Environment Canada's mercury site
- Health Canada's mercury site
- International Conference on Mercury as a Global Pollutant 2006-Madison, WI USA 2009-Guizhou, China 2011-Halifax, NS Canada
 |