Chemistry:Mercuric amidochloride
| |
| Names | |
|---|---|
| IUPAC name
Mercuric azanide chloride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Hg(NH 2)Cl | |
| Molar mass | 252.065 g/mol |
| Appearance | White crystalline solid or white amorphous powder[1] |
| Odor | Odorless[1] |
| Density | 5.56 g/cm3 |
| Boiling point | Sublimes[1] |
| 0.14 g in 100 mL of cold water 100 g in 100 mL of hot water (decomposes)[1] | |
| Solubility | Soluble in warm hydrochloric acid, nitric acid and acetic acid, insoluble in ethanol[1] |
| Pharmacology | |
| 1=ATC code }} | D08AK01 (WHO) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| HH300Script error: No such module "Preview warning".Category:GHS errors, HH310Script error: No such module "Preview warning".Category:GHS errors, HH330Script error: No such module "Preview warning".Category:GHS errors, HH373Script error: No such module "Preview warning".Category:GHS errors, HH410Script error: No such module "Preview warning".Category:GHS errors | |
| PP260Script error: No such module "Preview warning".Category:GHS errors, PP262Script error: No such module "Preview warning".Category:GHS errors, PP264Script error: No such module "Preview warning".Category:GHS errors, PP270Script error: No such module "Preview warning".Category:GHS errors, PP271Script error: No such module "Preview warning".Category:GHS errors, PP273Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP284Script error: No such module "Preview warning".Category:GHS errors, PP301+P316Script error: No such module "Preview warning".Category:GHS errors, PP302+P352Script error: No such module "Preview warning".Category:GHS errors, PP304+P340Script error: No such module "Preview warning".Category:GHS errors, PP316Script error: No such module "Preview warning".Category:GHS errors, PP319Script error: No such module "Preview warning".Category:GHS errors, PP320Script error: No such module "Preview warning".Category:GHS errors, PP321Script error: No such module "Preview warning".Category:GHS errors, PP330Script error: No such module "Preview warning".Category:GHS errors, PP361+P364Script error: No such module "Preview warning".Category:GHS errors, PP391Script error: No such module "Preview warning".Category:GHS errors, PP403+P233Script error: No such module "Preview warning".Category:GHS errors, PP405Script error: No such module "Preview warning".Category:GHS errors, PP501Script error: No such module "Preview warning".Category:GHS errors | |
| Flash point | Non-combustible |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mercuric amidochloride is an inorganic compound with the formula Hg(NH
2)Cl.
Preparation and properties
It arises from the reaction of mercury(II) chloride and ammonia (Calomel reaction), where the resulting mercuric amidochloride is highly insoluble.
- HgCl
2 + 2 NH
3 → HgCl(NH
2) + [NH
4]Cl
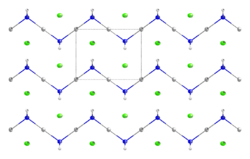
It forms white crystals in the shape of small prisms, which have earthy metallic taste.[1]It consists of a zig-zag 1-dimensional polymer (HgNH
2)
n with chloride counterions.[2][3]
It is stable in air, but darkens on exposure to light. Sublimes without melting at dull red heat.[1]
This substance is a deadly poison. If improperly handled, may cause dangerous environmental pollution, in soil, water bodies and air. When heated to decomposition, emits very toxic and irritating fumes of hydrogen chloride, nitrogen oxides, and mercury, thus, closed containers with this chemical may explode upon contact with heat. It is very toxic by inhalation, ingestion or contact with skin and its toxicity may be fatal. May cause dermatitis and psoriasis vulgaris. It is corrosive to the mucous membranes. It is not classified as a carcinogen in humans.[1]
Addition of base converts it into "Millon's base" (named after Eugène Millon), which has the formula Hg
2(OH)N · xH
2O. A variety of related amido and nitrido materials with chloride, bromide, and hydroxide are known.[4]
Uses
Before the toxicity of mercury was revealed, mercuric amidochloride, then known as "ammoniated mercury" or "white precipitate", was used as a topical skin antiseptic, especially impetigo, dermatomycosis and other certain dermatoses. It was also used for scaling in psoriasis, to treat pruritus ani, and against pinworm and ringworm infection (especially in dogs), against lesions on the body and near eyes, against crab louse infestation, against bumblefoot infection on poultry, and as a disinfectant.[1][5][6] Chronic use of this medication can lead to systemic mercury poisoning. Since less toxic medications are available now, to treat those conditions, there is no need to use mercuric amidochloride as a medication anymore.[1]
See also
- Merbromin, also known as "Mercurochrome", another antiseptic mercury compound
- Thiomersal, another antiseptic mercury compound
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Ammoniated mercury". https://pubchem.ncbi.nlm.nih.gov/compound/Ammoniated-mercury.
- ↑ Wells, A.F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. ISBN 0-19-855370-6.[page needed]
- ↑ Lipscomb, W. N. (1951). "The structure of mercuric amidochloride, HgNH2Cl". Acta Crystallographica 4 (3): 266–8. doi:10.1107/S0365110X51000866.
- ↑ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.[page needed]
- ↑ "Ammoniated mercury ointment: outdated but still in use". Contact Dermatitis 23 (3): 168–71. September 1990. doi:10.1111/j.1600-0536.1990.tb04778.x. PMID 2149317.
- ↑ "Mercury ammonium chloride". http://www.huidziekten.nl/allergie/stoffen/mercury-ammonium-chloride.htm.
 |


