Chemistry:OSU-03012
| |
| Names | |
|---|---|
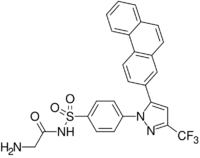
| IUPAC name
2-Amino-N-[4-[5-phenanthren-2-yl-3-(trifluoromethyl)pyrazol-1-yl]phenyl]acetamide
| |
| Other names
AR-12
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H19F3N4O | |
| Molar mass | 460.460 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
OSU-03012 (AR-12) is a celecoxib derivative with anticancer and anti-microbial activity. Unlike celecoxib, OSU-03012 does not inhibit COX, but inhibits several other important enzymes instead which may be useful in the treatment of some forms of cancer,[1][2] When combined with PDE5 inhibitors such as sildenafil or tadalafil, OSU-03012 was found to show enhanced anti-tumour effects in cell culture.[3]
Antimicrobial agent
- Antifungal activity against Candida albicans, non-albicans Candida spp., Cryptococcus neoformans, Blastomyces dermatitidis, Histoplasma, and Coccidioides via disruption of phosphoinositide-dependent kinase-1 and inhibition of acetyl—CoA synthetase activity.[4][5][6] Activity was enhanced with fluconazole co-delivery.[7]
- Antiparasitic activity against Leishmania donovani.[8][9]
- Antibacterial activity against Salmonella enterica,[10][11] and Francisella tularensis.[12][13] OSU-03012 has also illustrated sensitization of intracellular Salmonella enterica to aminoglycosides.[14]
- Antiviral when combined with PDE5 inhibitors such as sildenafil or tadalafil against a wide variety of organisms including Lassa virus, Marburg virus, and Ebola virus. This activity thought to be mediated via inhibition of the chaperone protein BiP.[15]
Antimicrobial activity is often not against the microbe directly (like conventional antibiotics), but rather host-directed activity that works on the cellular host to disrupt the pathogen within the cell.[16] Since the median lethal dose (LD50), in vitro, against macrophages is ~7 μM and near its effective concentration (EC50)[17][18] formulation of the compound has been shown to enhances its activity. For example, encapsulation of OSU-03012 into biodegradable polymeric (Acetalated dextran) micro/nanoparticles significantly reduces cytotoxicity, increases drug concentration per a cell, reduces toxicity in vivo, and facilitates needle-free delivery in vivo.[19][20]
Orphan drug designation
The European Commission has designated OSU-03012 as an orphan drug for use in combination with other drugs for treatment of two infections diseases, cryptococcosis and tularaemia. OSU-03012 received an orphan drug designation in combination with the antifungal drug fluconazole for cryptococcosis of the brain. It also received this designation for tularaemia in combination with the antibacterial drug gentamicin.[21]
References
- ↑ Booth, L; Cruickshanks, N; Ridder, T; Chen, CS; Grant, S; Dent, P (Dec 2012). "OSU-03012 interacts with lapatinib to kill brain cancer cells". Cancer Biology and Therapy 13 (14): 1501–11. doi:10.4161/cbt.22275. PMID 22990204.
- ↑ Ma, Y; McCarty, SK; Kapuriya, NP; Brendel, VJ; Wang, C; Zhang, X; Jarjoura, D; Saji, M et al. (Aug 2013). "Development of p21 activated kinase-targeted multikinase inhibitors that inhibit thyroid cancer cell migration". Journal of Clinical Endocrinology and Metabolism 98 (8): E1314–22. doi:10.1210/jc.2012-3937. PMID 23709653.
- ↑ Booth, L; Roberts, JL; Cruickshanks, N; Grant, S; Poklepovic, A; Dent, P (Oct 2014). "Regulation of OSU-03012 toxicity by ER stress proteins and ER stress-inducing drugs". Molecular Cancer Therapeutics 13 (10): 2384–98. doi:10.1158/1535-7163.MCT-14-0172. PMID 25103559.
- ↑ Liu, J; Qin, CK; Lv, W; Zhao, Q; Qin, CY (2013). "OSU-03012, a non-Cox inhibiting celecoxib derivative, induces apoptosis of human esophageal carcinoma cells through a p53/Bax/cytochrome c/caspase-9-dependent pathway". Anticancer Drugs 24 (7): 690–8. doi:10.1097/CAD.0b013e328362469f. PMID 23652278. http://pdfs.semanticscholar.org/f15a/a875946a47b389edfe401c4b613f3e8439ef.pdf.
- ↑ Koselny, K; Green, J; Favazzo, L; Glazier, VE; DiDone, L; Ransford, S; Krysan, DJ (8 Apr 2016). "Antitumor/Antifungal Celecoxib Derivative AR-12 is a Non-Nucleoside Inhibitor of the ANL-Family Adenylating Enzyme Acetyl CoA Synthetase". ACS Infect Dis 2 (4): 268–280. doi:10.1021/acsinfecdis.5b00134. PMID 27088128.
- ↑ Koselny, K; Green, J; DiDone, L; Halterman, JP; Fothergill, AW; Wiederhold, NP; Patterson, TF; Cushion, MT et al. (2016). "The Celecoxib Derivative AR-12 Has Broad-Spectrum Antifungal Activity In Vitro and Improves the Activity of Fluconazole in a Murine Model of Cryptococcosis". Antimicrob Agents Chemother 60 (12): 7115–7127. doi:10.1128/AAC.01061-16. PMID 27645246.
- ↑ Koselny, K; Green, J; DiDone, L; Halterman, JP; Fothergill, AW; Wiederhold, NP; Patterson, TF; Cushion, MT et al. (2016). "The Celecoxib Derivative AR-12 Has Broad-Spectrum Antifungal Activity In Vitro and Improves the Activity of Fluconazole in a Murine Model of Cryptococcosis". Antimicrob Agents Chemother 60 (12): 7115–7127. doi:10.1128/AAC.01061-16. PMID 27645246.
- ↑ Collier, MA; Peine, KJ; Gautum, S; Oghumu, S; Varikuti, S; Borteh, H; Papenfuss, TL; Satoskar, AR et al. (Feb 29, 2016). "Host-mediated Leishmania donovani treatment using AR-12 encapsulated in acetalated dextran microparticles". International Journal of Pharmaceutics 499 (1–2): 186–94. doi:10.1016/j.ijpharm.2016.01.004. PMID 26768723.
- ↑ Ainslie. "COMPOSITIONS AND METHODS FOR INHIBITING LEISHMANIA". http://www.freepatentsonline.com/y2016/0120844.html.
- ↑ Chiu, HC; Kulp, SK; Soni, S; Wang, D; Gunn, JS; Schlesinger, LS; Chen, CS (Dec 2009). "Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent.". Antimicrob Agents Chemother 53 (12): 5236–5244. doi:10.1128/aac.00555-09. PMID 19805568.
- ↑ Hoang, KV; Borteh, HM; Rajaram, MV; Peine, KJ; Curry, H; Collier, MA; Homsy, ML; Bachelder, EM et al. (Dec 2014). "Acetalated dextran encapsulated AR-12 as a host-directed therapy to control Salmonella infection.". Int J Pharm 477 (1–2): 334–343. doi:10.1016/j.ijpharm.2014.10.022. PMID 25447826.
- ↑ Chiu, HC; Soni, S; Kulp, SK; Curry, H; Wang, D; Gunn, JS; Schlesinger, LS; Chen, CS (Dec 2009). "Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent.". J Biomed Sci 16 (1): 110. doi:10.1186/1423-0127-16-110. PMID 20003180.
- ↑ Hoang, KV; Curry, H; Collier, MA; Borteh, H; Bachelder, EM; Schlesinger, LS; Gunn, JS; Ainslie, KM (Mar 25, 2016). "Needle-Free Delivery of Acetalated Dextran-Encapsulated AR-12 Protects Mice from Francisella tularensis Lethal Challenge". Antimicrob Agents Chemother 60 (4): 2052–62. doi:10.1128/AAC.02228-15. PMID 26787696.
- ↑ Lo, JH; Kulp, SK; Chen, CS; Chiu, HC (Dec 2014). "Sensitization of intracellular Salmonella enterica serovar Typhimurium to aminoglycosides in vitro and in vivo by a host-targeted antimicrobial agent". Antimicrob Agents Chemother 58 (12): 7375–82. doi:10.1128/AAC.03778-14. PMID 25267669.
- ↑ Dent, Paul (2014). "GRP78 / BiP / HSPA5 / Dna K is a universal therapeutic target for human disease". Journal of Cellular Physiology 230 (7): 1661–1676. doi:10.1002/jcp.24919. PMID 25546329.
- ↑ Collier, MA; Gallovic, MD; Peine, KJ; Duong, AD; Bachelder, EM; Gunn, JS; Schlesingr, LS; Ainslie, KM (Nov 2013). "Delivery of host cell-directed therapeutics for intracellular pathogen clearance.". Expert Rev Anti Infect Ther 11 (11): 1225–1235. doi:10.1586/14787210.2013.845524. PMID 24134600.
- ↑ Chiu, HC; Kulp, SK; Soni, S; Wang, D; Gunn, JS; Schlesinger, LS; Chen, CS (Dec 2009). "Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent.". Antimicrob Agents Chemother 53 (12): 5236–5244. doi:10.1128/aac.00555-09. PMID 19805568.
- ↑ Hoang, KV; Borteh, HM; Rajaram, MV; Peine, KJ; Curry, H; Collier, MA; Homsy, ML; Bachelder, EM et al. (Dec 2014). "Acetalated dextran encapsulated AR-12 as a host-directed therapy to control Salmonella infection.". Int J Pharm 477 (1–2): 334–343. doi:10.1016/j.ijpharm.2014.10.022. PMID 25447826.
- ↑ Hoang, KV; Borteh, HM; Rajaram, MV; Peine, KJ; Curry, H; Collier, MA; Homsy, ML; Bachelder, EM et al. (Dec 2014). "Acetalated dextran encapsulated AR-12 as a host-directed therapy to control Salmonella infection.". Int J Pharm 477 (1–2): 334–343. doi:10.1016/j.ijpharm.2014.10.022. PMID 25447826.
- ↑ Hoang, KV; Curry, H; Collier, MA; Borteh, H; Bachelder, EM; Schlesinger, LS; Gunn, JS; Ainslie, KM (Mar 25, 2016). "Needle-Free Delivery of Acetalated Dextran-Encapsulated AR-12 Protects Mice from Francisella tularensis Lethal Challenge". Antimicrob Agents Chemother 60 (4): 2052–62. doi:10.1128/AAC.02228-15. PMID 26787696.
- ↑ Arno Therapeutics Inc receives European orphan drug designation for OSU-03012 to treat two infectious diseases. Reuters, 30 Apr 2015.
 |

