Chemistry:Zotiraciclib
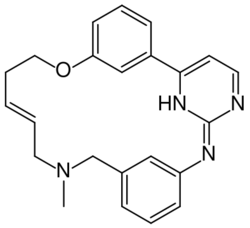 | |
| Clinical data | |
|---|---|
| Other names | TG02, SB1317 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >99%[1] |
| Metabolism | CYP3A4, CYP1A2[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H24N4O |
| Molar mass | 372.472 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zotiraciclib (TG02) is a potent oral spectrum selective[clarification needed][2] kinase inhibitor for the treatment of cancer. It was discovered in Singapore by S*BIO Pte Ltd and falls under the category of small molecule macrocycles. It crosses the blood brain barrier and acts by depleting Myc through the inhibition of cyclin-dependent kinase 9 (CDK9).[3] It is one of a number of CDK inhibitors under investigation; others targeting CDK9 for the treatment of acute myeloid leukemia include alvocidib and atuveciclib.[4][5] Myc overexpression is a known factor in many cancers, with 80 percent of glioblastomas characterized by this property.[6] Zotiraciclib has been granted orphan drug designation by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of gliomas.[7][8]
(As of January 2020), zotiraciclib is being evaluated by Adastra Pharmaceuticals in two separate Phase 1b clinical trials for the treatment of glioblastoma multiforme (GBM). Zotiracicib is also being developed as a potential treatment for diffuse intrinsic pontine glioma (DIPG), a rare pediatric cancer. Both forms of brain cancer are characterized by Myc overexpression.[6]
Development
The first Phase 1b clinical trial of zotiraciclib in GBM, sponsored by the National Cancer Institute (NCI), is a multi-arm, dose-finding study examining zotiraciclib plus dose-dense or metronomic temozolomide (TMZ) in adults with recurrent anaplastic astrocytoma and GBM.[9]
Zotiraciclib is also being explored for the treatment of DIPG, a rare pediatric cancer.[citation needed]
References
- ↑ 1.0 1.1 "Preclinical metabolism and pharmacokinetics of SB1317 (TG02), a potent CDK/JAK2/FLT3 inhibitor". Drug Metabolism Letters 6 (1): 33–42. March 2012. doi:10.2174/187231212800229336. PMID 22372550.
- ↑ "Discovery of kinase spectrum selective macrocycle (16E)-14-methyl-20-oxa-5,7,14,26-tetraazatetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8(27),9,11,16,21,23-decaene (SB1317/TG02), a potent inhibitor of cyclin dependent kinases (CDKs), Janus kinase 2 (JAK2), and fms-like tyrosine kinase-3 (FLT3) for the treatment of cancer". Journal of Medicinal Chemistry 55 (1): 169–96. January 2012. doi:10.1021/jm201112g. PMID 22148278.
- ↑ "Novel Targeting of Transcription and Metabolism in Glioblastoma". Clinical Cancer Research 24 (5): 1124–1137. March 2018. doi:10.1158/1078-0432.CCR-17-2032. PMID 29254993.
- ↑ "Cyclin-dependent kinase inhibitors for the treatment of chronic lymphocytic leukemia". Seminars in Oncology 43 (2): 265–73. April 2016. doi:10.1053/j.seminoncol.2016.02.003. PMID 27040705.
- ↑ "Current and emerging therapies for patients with acute myeloid leukemia: a focus on MCL-1 and the CDK9 pathway". The American Journal of Managed Care 24 (16 Suppl): S356–S365. August 2018. PMID 30132679.
- ↑ 6.0 6.1 "Myc inhibition is effective against glioma and reveals a role for Myc in proficient mitosis". Nature Communications 5 (4632): 4632. August 2014. doi:10.1038/ncomms5632. PMID 25130259. Bibcode: 2014NatCo...5.4632A.
- ↑ "FDA grants orphan drug designation to zotiraciclib for the treatment of glioma". Center for Cancer Research (Cancer Research Center). 2020-01-09. https://ccr.cancer.gov/news/article/fda-grants-orphan-drug-designation-to-zotiraciclib-for-the-treatment-of-glioma.
- ↑ "EU/3/19/2202". 2020-01-21. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3192202.
- ↑ Clinical trial number NCT02942264 for "Zotiraciclib (TG02) Plus Dose-Dense or Metronomic Temozolomide Followed by Randomized Phase II Trial of Zotiraciclib (TG02) Plus Temozolomide Versus Temozolomide Alone in Adults With Recurrent Anaplastic Astrocytoma and Glioblastoma" at ClinicalTrials.gov
 |

