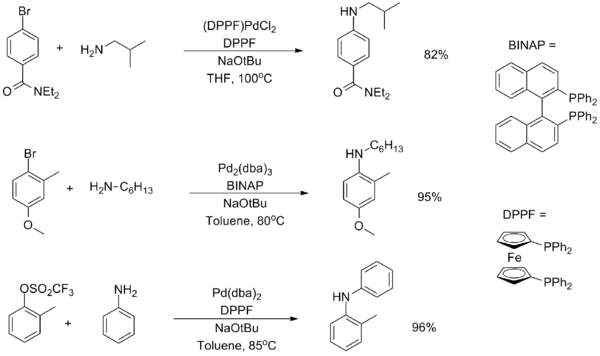
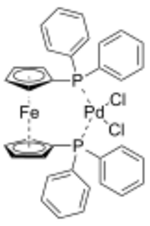
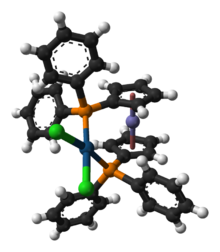
Chemistry:(1,1'-Bis(diphenylphosphino)ferrocene)palladium(II) dichloride
From HandWiki
Short description: Chemical compound
| |
| |
| Names | |
|---|---|
| IUPAC name
[1,1'-Bis(diphenylphosphino)ferrocene-κ2P,P']dichloropalladium(II)
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C34H28Cl2FeP2Pd | |
| Molar mass | 731.71 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
[1,1'‑Bis(diphenylphosphino)ferrocene]palladium(II) dichloride is a palladium complex containing the bidentate ligand 1,1'-bis(diphenylphosphino)ferrocene (dppf), abbreviated as [(dppf)PdCl2]. This commercially available material can be prepared by reacting dppf with a suitable nitrile complex of palladium dichloride:[1]
The compound is popularly used for palladium-catalyzed coupling reactions,[2][3] such as the Buchwald–Hartwig amination[4] and the reductive homocoupling of aryl halides.[5]
References
- ↑ Nataro, Chip; Fosbenner, Stephanie M. (2009). "Synthesis and Characterization of Transition-Metal Complexes Containing 1,1'-Bis(diphenylphosphino)ferrocene". J. Chem. Educ. 86 (12): 1412–1415. doi:10.1021/ed086p1412. Bibcode: 2009JChEd..86.1412N.
- ↑ Gildner, Peter G.; Colacot, Thomas J. (2015). "Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings". Organometallics 34 (23): 5497–5508. doi:10.1021/acs.organomet.5b00567.
- ↑ Li, Jie Jack; Limberakis, Chris; Pflum, Derek A. (2007). "Carbon–Carbon Bond Formation". Modern Organic Synthesis in the Laboratory: A Collection of Standard Experimental Procedures. Oxford University Press. pp. 111–. ISBN 9780198040637. https://books.google.com/books?id=kTQNYINDXpkC&q=Pd(dppf)Cl2&pg=PA3.
- ↑ John P. Wolfe; Seble Wagaw; Stephen L. Buchwald (1996). "An Improved Catalyst System for Aromatic Carbon-Nitrogen Bond Formation: The Possible Involvement of Bis(Phosphine) Palladium Complexes as Key Intermediates". J. Am. Chem. Soc. 118 (30): 7215–7216. doi:10.1021/ja9608306.
- ↑ Zeng, Minfeng; Du, Yijun; Shao, Linjun; Qi, Chenze; Zhang, Xian-Man (2010). "Palladium-Catalyzed Reductive Homocoupling of Aromatic Halides and Oxidation of Alcohols". J. Org. Chem. 75 (8): 2556–2563. doi:10.1021/jo100089d. PMID 20302294.
 |