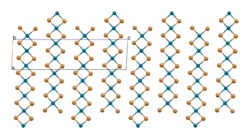Chemistry:Palladium(II) bromide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Br2Pd | |
| Molar mass | 266.228 g/mol |
| Related compounds | |
Other anions
|
Palladium(II) fluoride Palladium(II) chloride Palladium(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Palladium(II) bromide is an inorganic compound of palladium and bromine with the chemical formula PdBr2. It is a commercially available, although less common than palladium(II) chloride, the usual entry point to palladium complexes. It is a diamagnetic solid.
Structure
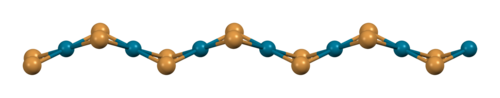
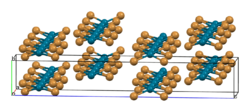
As confirmed by X-ray crystallography, PdBr2 is a coordination polymer.[1] It crystallises in the P21/c space group and the structure consists of wavy ribbons of edge-sharing PdBr4 coordination squares.[2]
Reactions
Palladium(II) bromide is insoluble in water but dissolves when heated in acetonitrile to give monomeric acetonitrile adducts:[3]
- PdBr2 + 2 MeCN → PdBr2(MeCN)2
PdBr2 exhibits many of the properties of palladium chloride and palladium acetate, giving catalysts active for carbonylations and cross-coupling reactions.[4]
References
- ↑ K. Brodersen, G. Thiele, H. Gaedcke (1966). "Die Konstitution des Palladium(II)-bromids". Z. Anorg. Allg. Chem. 348 (3–4): 162–167. doi:10.1002/zaac.19663480307.
- ↑ "Information card for entry 1534319". 1966. https://www.crystallography.net/cod/1534319.html.
- ↑ O. A. Zalevskaya, E. G. Vorob'eva1, I. A. Dvornikova and A. V. Kuchin (2008). "Palladium Complexes Based on Optically Active Terpene Derivatives of Ethylenediamine". Russian Journal of Coordination Chemistry 34 (11): 855–857. doi:10.1134/S1070328408110110.
- ↑ Mahoney, Stuart J.; Fillion, Eric (2013). "Palladium(II) Bromide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01617. ISBN 978-0471936237.
 |