Chemistry:Palladium(II) fluoride
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| F2Pd | |
| Molar mass | 144.42 g·mol−1 |
| Appearance | pale violet crystalline solid; hygroscopic[1] |
| Density | 5.76 g cm−3[1] |
| Melting point | 952 °C (1,746 °F; 1,225 K)[1] |
| reacts with water | |
| Structure | |
| tetragonal | |
| octahedral | |
| Related compounds | |
Other anions
|
Palladium(II) chloride Palladium(II) bromide Palladium(II) iodide |
Other cations
|
Nickel(II) fluoride Platinum(II) fluoride Platinum(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Palladium(II) fluoride, also known as palladium difluoride, is the chemical compound of palladium and fluorine with the formula PdF2.
Synthesis
PdF2 is prepared by refluxing palladium(II,IV) fluoride, PdII[PdIVF6], with selenium tetrafluoride, SeF4.
- Pd[PdF6] + SeF4 → 2PdF2 + SeF6
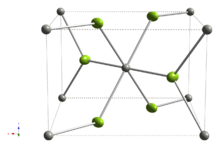
Structure and paramagnetism
Like its lighter congener nickel(II) fluoride, PdF2 adopts a rutile-type crystal structure,[2][3] containing octahedrally coordinated palladium, which has the electronic configuration t62g e2g. This configuration causes PdF2 to be paramagnetic[4] due to two unpaired electrons, one in each eg-symmetry orbital of palladium.
Applications
Palladium fluoride is an insoluble powder used in infrared optical sensors,[5] and in situations where reactivity to oxygen makes palladium oxide unsuitable.
See also
References
- ↑ 1.0 1.1 1.2 CRC Handbook, 89th edition
- ↑ Bachmann, B.; Müller, B. G. (1993). "Einkristalluntersuchungen an Fluoroperowskiten MPdF3 (M = Rb, K) und PdF2". Z. Anorg. Allg. Chem. 619 (2): 387–391. doi:10.1002/zaac.19936190225.
- ↑ Holleman, A. F.; Wiberg, Egon; Wiberg, Nils (2001) (in en). Inorganic Chemistry. Web: Academic Press. p. 1515. ISBN 9780123526519. https://books.google.com/books?id=Mtth5g59dEIC&pg=PA439. Retrieved 30 May 2020.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1152–1153. ISBN 978-0-08-037941-8.
- ↑ "American_Elements.com". https://www.americanelements.com/palladium-fluoride-13444-96-7.
 |

