Chemistry:Cinnamic acid
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-3-Phenylprop-2-enoic acid | |
| Systematic IUPAC name
Cinnamic acid | |
| Other names
trans-Cinnamic acid
Phenylacrylic acid[1] Cinnamylic acid 3-Phenylacrylic acid (E)-Cinnamic acid Benzenepropenoic acid Isocinnamic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1905952 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 3731 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H8O2 | |
| Molar mass | 148.161 g·mol−1 |
| Appearance | White monoclinic crystals |
| Odor | Honey-like[2] |
| Density | 1.2475 g/cm3[3] |
| Melting point | 133 °C (271 °F; 406 K)[3] |
| Boiling point | 300 °C (572 °F; 573 K)[3] |
| 500 mg/L[3] | |
| Acidity (pKa) | 4.44 |
| −7.836×10−5 cm3/mol | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | > 100 °C (212 °F; 373 K)[3] |
| Related compounds | |
Related compounds
|
Benzoic acid, Phenylacetic acid, Phenylpropanoic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents.[4] Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.[5]
Occurrence and production
Biosynthesis
Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine.[6]
Natural occurrence
It is obtained from oil of cinnamon, or from balsams such as storax.[4] It is also found in shea butter.[citation needed] Cinnamic acid has a honey-like odor;[2] and its more volatile ethyl ester, ethyl cinnamate, is a flavor component in the essential oil of cinnamon, in which related cinnamaldehyde is the major constituent. It is also found in wood from all species of trees.[7]
Synthesis
Cinnamic acid was first synthesized by the base-catalysed condensation of acetyl chloride and benzaldehyde, followed by hydrolysis of the acid chloride product.[5] In 1890, Rainer Ludwig Claisen described the synthesis of ethyl cinnamate via the reaction of ethyl acetate with benzaldehyde in the presence of sodium as base.[8] Another way of preparing cinnamic acid is by the Knoevenagel condensation reaction.[9] The reactants for this are benzaldehyde and malonic acid in the presence of a weak base, followed by acid-catalyzed decarboxylation. It can also be prepared by oxidation of cinnamaldehyde, condensation of benzal chloride and sodium acetate (followed by acid hydrolysis), and the Perkin reaction. The oldest commercially used route to cinnamic acid involves the Perkin reaction, which is given in the following scheme[5]
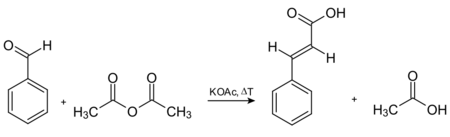 Synthesis of cinnamic acid via the Perkin reaction.[10]
Synthesis of cinnamic acid via the Perkin reaction.[10]
Metabolism
Cinnamic acid, obtained from autoxidation of cinnamaldehyde, is metabolized into sodium benzoate in the liver.[11]
Uses
Cinnamic acid is used in flavorings, synthetic indigo, and certain pharmaceuticals. A major use is as a precursor to produce methyl cinnamate, ethyl cinnamate, and benzyl cinnamate for the perfume industry.[4] Cinnamic acid is a precursor to the sweetener aspartame via enzyme-catalysed amination to give phenylalanine.[5] Cinnamic acid can dimerize in non-polar solvents resulting in different linear free energy relationships.[12]
References
- ↑ "Cinnamic Acid". Encyclopædia Britannica. 6 (11th ed.). 1911. p. 376.
- ↑ 2.0 2.1 "Cinnamic acid". http://www.flavornet.org/info/140-10-3.html.
- ↑ 3.0 3.1 3.2 3.3 3.4 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ 4.0 4.1 4.2 Budavari, Susan, ed. (1996), An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.), Merck, ISBN 0911910123
- ↑ 5.0 5.1 5.2 5.3 Garbe, Dorothea (2012). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a07_099.
- ↑ Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant 3 (1): 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
- ↑ Oldach, Laurel (February 22, 2023). "Forensic researchers use mass spectrometry to identify smuggled wood". American Chemical Society. https://cen.acs.org/analytical-chemistry/mass-spectrometry/Forensic-researchers-use-mass-spectrometry-to-identify-smuggled-wood/101/i7.
- ↑ Claisen, L. (1890). "Zur Darstellung der Zimmtsäure und ihrer Homologen". Berichte der Deutschen Chemischen Gesellschaft 23: 976–978. doi:10.1002/cber.189002301156. https://babel.hathitrust.org/cgi/pt?id=uc1.b3481786;view=1up;seq=992.
- ↑ Tieze, L. (1988). Reactions and Synthesis in the Organic Chemistry Laboratory. Mill Vall, CA. p. 1988.
- ↑ F. K. Thayer (1925). "m-Nitrocinnamic Acid". Organic Syntheses 5: 83. doi:10.15227/orgsyn.005.0083.
- ↑ "Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders". Journal of Neuroimmune Pharmacology 8 (3): 739–55. June 2013. doi:10.1007/s11481-013-9447-7. PMID 23475543.
- ↑ Bradley, J.-C.; Abraham, M. H.; Acree, W. E.; Lang, A.; Beck, S. N.; Bulger, D. A.; Clark, E. A.; Condron, L. N. et al. (2015). "Determination of Abraham model solute descriptors for the monomeric and dimeric forms of trans-cinnamic acid using measured solubilities from the Open Notebook Science Challenge". Chemistry Central Journal 9: 11. doi:10.1186/s13065-015-0080-9. PMID 25798191.
 |


