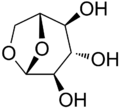
Chemistry:Levoglucosan
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1R,2S,3S,4R,5R)-6,8-Dioxabicyclo[3.2.1]octane-2,3,4-triol | |
| Other names
1,6-Anhydro-beta-glucopyranose
Leucoglucosan | |
| Identifiers | |
3D model (JSmol)
|
|
| 80998 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | 1,6-Anhydro-beta-glucopyranose |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10O5 | |
| Molar mass | 162.141 g·mol−1 |
| Appearance | Colorless crystals |
| Density | 1.688 g·cm−3 (predicted) |
| Melting point | 182 to 184 °C (360 to 363 °F; 455 to 457 K) |
| Boiling point | 384 °C (723 °F; 657 K) predicted |
| log P | -0.04 (predicted) |
| Vapor pressure | 24.1 μPa (predicted) |
| Hazards | |
| Flash point | 185.9 °C (366.6 °F; 459.0 K) (predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Levoglucosan (C6H10O5) is an organic compound with a six-carbon ring structure formed from the pyrolysis of carbohydrates, such as starch and cellulose.[1] As a result, levoglucosan is often used as a chemical tracer for biomass burning in atmospheric chemistry studies, particularly with respect to airborne particulate matter. Along with other tracers such as potassium, oxalate, and gaseous acetonitrile,[2] levoglucosan has been shown to be highly correlated with regional fires. This is because the gas emitted by the pyrolysis of wood (biomass) contains significant amounts of levoglucosan.
Levoglucosan has been described as "an unequivocal biomass burning tracer" in the context of forest and brush fires.[3] But the anhydrosugar has only been found detectable in low temperature samples (150-350 °C), meaning that its value as an indicator for smoke from controlled biomass combustion in, for instance, modern domestic wood stoves which operate at temperatures above 500 °C, is "very doubtful".[4] Levoglucosan is a marker for coal combustion as well as wood.[5]
The hydrolysis of levoglucosan generates the fermentable sugar glucose. Levoglucosan can be utilized in the synthesis of chiral polymers such as unhydrolysable glucose polymers.
References
- ↑ Lakshmanan, Chambra M.; Hoelscher, Harold E. (1970). "Production of Levoglucosan by Pyrolysis of Carbohydrates. Pyrolysis in Hot Inert Gas Stream". Industrial & Engineering Chemistry Product Research and Development 9: 57–59. doi:10.1021/i360033a011.
- ↑ Aiken, A. C.; De Foy, B.; Wiedinmyer, C.; Decarlo, P. F.; Ulbrich, I. M.; Wehrli, M. N.; Szidat, S.; Prevot, A. S. H. et al. (2010). "Mexico city aerosol analysis during MILAGRO using high resolution aerosol mass spectrometry at the urban supersite (T0) – Part 2: Analysis of the biomass burning contribution and the non-fossil carbon fraction". Atmospheric Chemistry and Physics 10 (12): 5315. doi:10.5194/acp-10-5315-2010. Bibcode: 2010ACP....10.5315A. https://boris.unibe.ch/5173/1/acp-10-5315-2010.pdf.
- ↑ "Identification of biomass burning tracers". Euro 'Milestone' Survey. http://airuse.eu/wp-content/uploads/2013/11/B4-2MILESTONE-tracers-biomass.pdf. Retrieved 20 March 2015.
- ↑ Harrison, Roy M.; Beddows, David C.S.; Jones, Alan M.; Calvo, Ana; Alves, Célia; Pio, Casimiro (2013). "An evaluation of some issues regarding the use of aethalometers to measure woodsmoke concentrations". Atmospheric Environment 80: 540–548. doi:10.1016/j.atmosenv.2013.08.026. Bibcode: 2013AtmEn..80..540H. http://pure-oai.bham.ac.uk/ws/files/16250444/An_Evaluation_of_Some_Issues_PostPrint.pdf.
- ↑ Yan, Caiqing; Zheng, Mei; Sullivan, Amy P.; Shen, Guofeng; Chen, Yingjun; Wang, Shuxiao; Zhao, Bin; Cai, Siyi et al. (2018). "Residential Coal Combustion as a Source of Levoglucosan in China". Environmental Science & Technology 52 (3): 1665–1674. doi:10.1021/acs.est.7b05858. PMID 29244948. Bibcode: 2018EnST...52.1665Y.
 |

