Chemistry:Potassium niobate
| |
| Names | |
|---|---|
| IUPAC name
Potassium niobate
| |
| Other names
niobate, niobium potassium oxide, potassium columbate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| KNbO3 | |
| Molar mass | 180.003 g·mol−1 |
| Appearance | White rhombohedral crystals |
| Density | 4.640 g/cm3 |
| Melting point | ≈ 1100 °C[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3000 mg/kg (oral, rat) |
| Related compounds | |
Other anions
|
Potassium chlorate Potassium bromate |
Other cations
|
Lithium niobate Strontium barium niobate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium niobate (KNbO3) is an inorganic compound with the formula KNbO3. A colorless solid, it is classified as a perovskite ferroelectric material.[2] It exhibits nonlinear optical properties, and is a component of some lasers.[3] Nanowires of potassium niobate have been used to produce tunable coherent light.
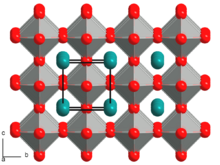
Structure
On cooling from high temperature, KNbO3 undergoes a series of structural phase transitions. At 435 °C, the crystal symmetry changes from cubic centrosymmetric (Pm3m) to tetragonal non-centrosymmetric (P4mm). On further cooling, at 225 °C the crystal symmetry changes from tetragonal (P4mm) to orthorhombic (Amm2) and at −50 °C from orthorhombic (Amm2) to rhombohedral (R3m).
Applications and research
In addition to research in electronic memory storage,[4] potassium niobate is used in resonant doubling.[5] This technique allows small infrared lasers to convert output into blue light, a critical technology for the production of blue lasers and technology dependent upon them.
Potassium niobate has been found useful in many different areas of materials science,[4] including properties of lasers,[5] quantum teleportation,[6] and it has been used to study the optical properties of particulate composite materials.[7]
Safety
The -1">50 for potassium niobate is 3000 mg/kg (oral, rat).
References
- ↑ CRC Handbook, 90th Edition (03 Jun 2009) ISBN:1-4200-9084-4, section 4: Physical Constants of Inorganic Compounds, page 83
- ↑ Hewat, A W (1973-03-28). "Soft modes and the structure, spontaneous polarization and Curie constants of perovskite ferroelectrics: tetragonal potassium niobate" (in en). Journal of Physics C: Solid State Physics 6 (6): 1074–1084. doi:10.1088/0022-3719/6/6/020. ISSN 0022-3719. Bibcode: 1973JPhC....6.1074H. https://iopscience.iop.org/article/10.1088/0022-3719/6/6/020/meta.
- ↑ Palik, Edward D. (1998). Handbook of Optical Constants of Solids 3. Academic Press. p. 821. ISBN 978-0-12-544423-1. https://books.google.com/books?id=VpuVttd2cuEC&pg=PA821. Retrieved 13 December 2012.
- ↑ 4.0 4.1 "In Science Fields". The Science News-Letter 62 (17): 264–265. 1952-10-25. doi:10.2307/3931381.
- ↑ 5.0 5.1 Regalado, Antonio (1995-03-31). "Blue-Light Special". Science. New Series 267 (5206): 1920. doi:10.1126/science.267.5206.1920. PMID 17770099. Bibcode: 1995Sci...267.1920R.
- ↑ Furusawa, A.; J. L. Sørensen; S. L. Braunstein; C. A. Fuchs; H. J. Kimble; E. S. Polzik (1998-10-23). "Unconditional Quantum Teleportation". Science. New Series 282 (5389): 706–709. doi:10.1126/science.282.5389.706. PMID 9784123. Bibcode: 1998Sci...282..706F.(Subscription content?)
- ↑ Lakhtakia, Akhlesh; Tom G. Mackay (2007-02-08). "Electrical Control of the Linear Optical Properties of Particulate Composite Materials". Proceedings of the Royal Society A 463 (2078): 583–592. doi:10.1098/rspa.2006.1783. Bibcode: 2007RSPSA.463..583L.
 |


