Chemistry:Niobium(IV) fluoride
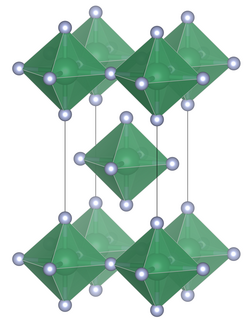 Unit cell of niobium(IV) fluoride.
| |
| Names | |
|---|---|
| IUPAC name
Niobium(IV) fluoride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| NbF 4 | |
| Molar mass | 168.9 g/mol |
| Appearance | black solid |
| Melting point | 350 °C (662 °F; 623 K) (decomposes) |
| Structure[1] | |
| tetragonal | |
| I4/mmm | |
a = 4,0876(5) Å, c = 8,1351(19) Å
| |
| [6]Nb | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Niobium(IV) fluoride is a chemical compound with the formula NbF
4. It is a nonvolatile black solid.
Properties
NbF
4 absorbs vapor strongly and turns into NbO
2F in moist air. It reacts with water to form a brown solution and a brown precipitate whose components are unknown. It is stable between 275 °C and 325 °C when heated in a vacuum. However, it disproportionates at 350 °C rapidly to form niobium(V) fluoride and niobium(III) fluoride:[2]
- 2 NbF
4 → NbF
5 + NbF
3 (at 350 °C)
Structure
Niobium(IV) fluoride adopts a crystal structure analogous to that of tin(IV) fluoride, in which each niobium atom is surrounded by six fluorine atoms forming an octahedron. Of the six fluorine atoms surrounding a single niobium atom, four are bridging to adjacent octahedra, leading to a structure of octahedra connected in layers.[1]
References
- ↑ 1.0 1.1 Bandemehr, Jascha; Conrad, Matthias; Kraus, Florian (29 July 2016). "Redetermination of the crystal structure of NbF4". Acta Crystallographica Section E 72 (8): 1211–1213. doi:10.1107/S2056989016012081. PMID 27536416.
- ↑ 张青莲 (1981). 无机化学丛书. Beijing: Science Press. pp. 323. ISBN 7-03-002238-6.
 |

