Chemistry:Niobocene dichloride
| |
| Names | |
|---|---|
| IUPAC name
Dichloridobis (η5-cyclopentadienyl)niobium
| |
| Other names
Niobocene dichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| |
| |
| Properties | |
| C10H10Cl2Nb | |
| Molar mass | 294 g/mol |
| Appearance | brown solid |
| Melting point | dec. |
| Boiling point | dec. |
| soluble (hydrolysis) | |
| Solubility in other solvents | sparingly in chlorocarbons |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Cp2TiCl2 Cp2MoCl2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
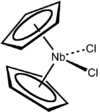
Niobocene dichloride is the organometallic compound with the formula (C5H5)2NbCl2, abbreviated Cp2NbCl2. This paramagnetic brown solid is a starting reagent for the synthesis of other organoniobium compounds. The compound adopts a pseudotetrahedral structure with two cyclopentadienyl and two chloride substituents attached to the metal. A variety of similar compounds are known, including Cp2TiCl2.
Preparation and structure
It was originally reported by Geoffrey Wilkinson.[2] It is prepared via a multistep reaction beginning with treatment of niobium pentachloride with cyclopentadienylsodium:[3]
- NbCl5 + 6 NaC5H5 → 5 NaCl + (C5H5)4Nb + organic products
- (C5H5)4Nb + 2 HCl + 0.5 O2 → [{C5H5)2NbCl}2O]Cl2 + 2 C5H6
- 2 HCl + [{(C5H5)2NbCl}2O]Cl2 + SnCl2 → 2 (C5H5)2NbCl2 + SnCl4 + H2O
The compound adopts a "clamshell" structure characteristic of a bent metallocene where the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being about 130.3°. The Cl-Nb-Cl angle of 85.6° is narrower than in zirconocene dichloride (97.1°) but wider than in molybdocene dichloride (82°). This trend is consistent with the orientation of the HOMO in this class of complex.[4]
Applications and further work
Unlike the related zirconacene and titanocene dichlorides, no applications have been found for this compound, although it has been studied widely. It was investigated as a potential anti-cancer agent.[5]
References
- ↑ "Bis(cyclopentadienyl)niobium(IV) dichloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/16211739#section=Safety-and-Hazards.
- ↑ Wilkinson, G.; Birmingham, J. G. (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- ↑ C. R. Lucas (2007). "Dichlorobis(η 5 ‐Cyclopentadienyl) Niobium(IV)". Inorganic Syntheses. 28. 267–270. doi:10.1002/9780470132593.ch68. ISBN 978-0-471-52619-3.
- ↑ K. Prout, T. S. Cameron, R. A. Forder, and in parts S. R. Critchley, B. Denton and G. V. Rees "The crystal and molecular structures of bent bis-π-cyclopentadienyl-metal complexes: (a) bis-π-cyclopentadienyldibromorhenium(V) tetrafluoroborate, (b) bis-π-cyclopentadienyldichloromolybdenum(IV), (c) bis-π-cyclopentadienylhydroxomethylaminomolybdenum(IV) hexafluorophosphate, (d) bis-π-cyclopentadienylethylchloromolybdenum(IV), (e) bis-π-cyclopentadienyldichloroniobium(IV), (f) bis-π-cyclopentadienyldichloromolybdenum(V) tetrafluoroborate, (g) μ-oxo-bis[bis-π-cyclopentadienylchloroniobium(IV)] tetrafluoroborate, (h) bis-π-cyclopentadienyldichlorozirconium" Acta Crystallogr. 1974, volume B30, pp. 2290–2304. doi:10.1107/S0567740874007011
- ↑ Mokdsi, G.; Harding, M. M. (2001). "A1H NMR study of the Interaction of Antitumor Metallocenes with Glutathione". J. Inorg. Biochem. 86 (2–3): 611–616. doi:10.1016/S0162-0134(01)00221-5. PMID 11566334.
 |
