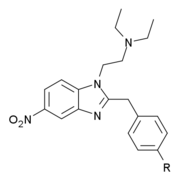
Chemistry:List of benzimidazole opioids
Benzimidazole opioids, also known as nitazenes, are a class of synthetic opioids with an unusual benzimidazole structure often referred to as opioid New Psychoactive Substances (opioid NPS).[1] First synthesized in the 1950s by CIBA Pharmaceuticals as potential analgesic medications, several substances in the class have been identified, the best known being etonitazene. Like other synthetic opioids, benzimidazole opioids bind the mu-opioid receptor and may exhibit potency up to several hundred times that of morphine. While several substances in this class have found applications in research, they have never been used in clinical medicine due to their profound risk of respiratory depression and death,[2] and have recently been recognized as emerging drugs of abuse.[3][4][5] Isotonitazine was first identified in samples of illicit drugs, and implicated in opioid overdose deaths in Europe, Canada, and the United States beginning in 2019.[6] Previously known nitazene analogs such as metonitazine and butonitazine, as well as novel nitazenes not previously patented, have since been discovered in toxicologic samples during forensic investigations.[5]
The structure-activity relationship of the drug class has been explored to a reasonable extent. The optimal substitution pattern is fairly tightly defined (i.e. N,N-diethyl on the amine nitrogen, 4-ethoxy on the benzyl ring and 5-nitro on the benzimidazole ring), but even derivatives incorporating only some of these features are still potent opioids. If a methyl or carboxamide group is added on the alpha carbon of the benzyl group, or the benzyl is replaced by 2-phenylethyl, compounds of similar activity are obtained. Relative analgesic activity values are derived from tests on mice and cannot be extrapolated directly to humans, though the same general activity trends apply.[7][8][9][10][11][12][13][14][15][16][17][18][19][excessive citations]
A 2019 publication[20] has shown the possibility the previously assumed binding position of the benzimidazole class,[21] acting as a semi-rigid fentanyl analogue may be incorrect. Based on a large scale analysis of known opioid receptor ligands a template was created through manual overlaying and alignment which has identified several mu-specific areas within the receptor. In this analysis, it is noted, etonitazene now more closely matches another, separate mu-specific region, sharing only a small area in common with the fentanyl class.
Table of benzimidazole opioids
| Chemical structure | Drug name | Ring substitution | Analgesic potency (morphine = 1) | PubChem | CAS number |
|---|---|---|---|---|---|
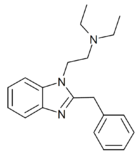
|
1-diethylaminoethyl-2-benzyl-benzimidazole | hydrogen | 0.1 | 28787 | 17817-67-3 |
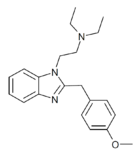
|
Metodesnitazene (Metazene) | 4-methoxy | 1 | 26412 | 14030-77-4 1071546-40-1 (HCl) |
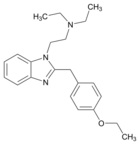
|
Etodesnitazene (Etazene) | 4-ethoxy | 70 | 149797386 | 14030-76-3 |
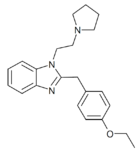
|
Etodesnitazene pyrrolidine analogue | 4-ethoxy | 20 | 162623599 | |
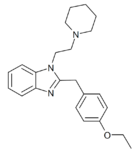
|
Etodesnitazene piperidine analogue | 4-ethoxy | 10 | 162623611 | 102762-98-1 |
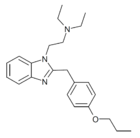
|
Protodesnitazene | 4-(n-propoxy) | 10 | 157010653 | 805212-21-9 |
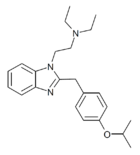
|
Isotodesnitazene | 4-isopropoxy | ~75 | 162623708 | 2732926-27-9 |
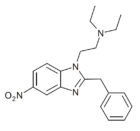
|
Nitazene | hydrogen | 2 | 15327524 | |
|
meta-Metonitazene | 3-methoxy | 2 | ||
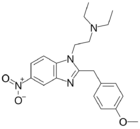
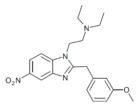
|
Metonitazene | 4-methoxy | 100 | 53316366 | 14680-51-4 |
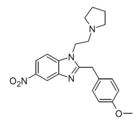
|
Metonitazepyne | 4-methoxy | |||
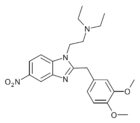
|
Dimetonitazene | 3,4-dimethoxy | 10 | 162623836 | |
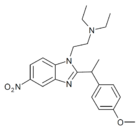
|
α-methyl-metonitazene | 4-methoxy | 50 | 162625089 | 806634-80-0 |
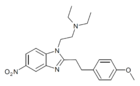
|
Metonitazene phenethyl homologue | 4-methoxy | 50 | ||
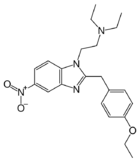
|
Etonitazene | 4-ethoxy | 1000 | 13493 | 911-65-9 |
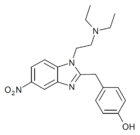
|
O-Desethyl-etonitazene | 4-hydroxy | 1 | 156588969 | 94758-81-3 |
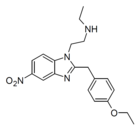
|
N-Desethyl-etonitazene | 4-ethoxy | 162623580 | 2732926-26-8 | |
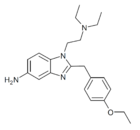
|
Etonitazene 5-amino metabolite | 4-ethoxy | 2 | 13408927 | |

|
Etomethazene | 4-ethoxy | |||
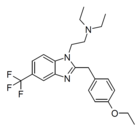
|
Etonitazene 5-trifluoromethyl analogue[22] | 4-ethoxy | 21815908 | ||
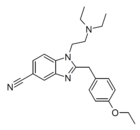
|
Etonitazene 5-cyano analogue [23] | 4-ethoxy | 27268 | 15419-87-1 | |
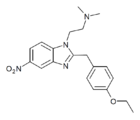
|
Etonitazene N,N-dimethyl analogue | 4-ethoxy | 20 | 67089584 | 714190-52-0 |
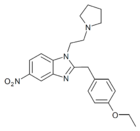
|
Etonitazepyne | 4-ethoxy | 155804760 | 2785346-75-8 | |
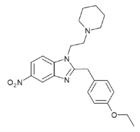
|
Etonitazepipne | 4-ethoxy | 100 | 162623834 | 734496-28-7 |
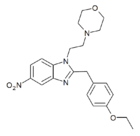
|
Etonitazene morpholine analogue | 4-ethoxy | 2 | 162623685 | 805958-08-1 |
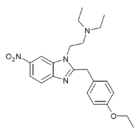
|
Etonitazene 6-nitro isomer (iso-etonitazene) [24] | 4-ethoxy | 20 | 59799752 | 114160-61-1 |
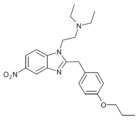
|
Protonitazene | 4-(n-propoxy) | 200 | 156589001 | 119276-01-6 95958-84-2 |
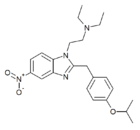
|
Isotonitazene | 4-isopropoxy | 500 | 145721979 | 14188-81-9 |
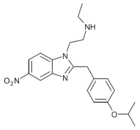
|
N-desethyl-isotonitazene | 4-isopropoxy | ~1000 | 162623899 | 2732926-24-6 |
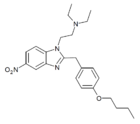
|
Butonitazene | 4-butoxy | 5 | 156588955 | 95810-54-1 |
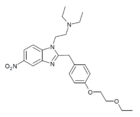
|
Etoetonitazene | 4-ethoxyethoxy | 50 | 162623504 | 806642-21-7 |
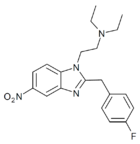
|
Fluonitazene | 4-fluoro | 1 | 156588967 | 2728-91-8 |
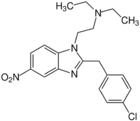
|
Clonitazene | 4-chloro | 3 | 62528 | 3861-76-5 |
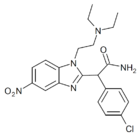
|
α-carboxamido-clonitazene | 4-chloro | 3 | ||
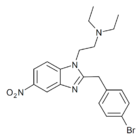
|
Bronitazene | 4-bromo | 5 | 162623726 | |
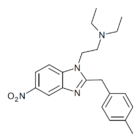
|
Methylnitazene (Menitazene) | 4-methyl | 10 | 162623683 | 95282-00-1 |
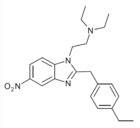
|
Ethylnitazene (Enitazene) | 4-ethyl | 20 | 162623845 | |
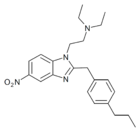
|
Propylnitazene (Pronitazene) | 4-propyl | 50 | 162623877 | 700342-00-3 |
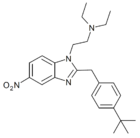
|
t-Butylnitazene | 4-(tert-butyl) | 2 | 162623621 | 805215-64-9 |
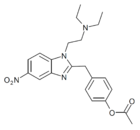
|
Acetoxynitazene | 4-acetoxy | 5 | 162623779 | 102760-24-7 |
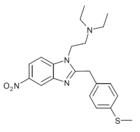
|
Methylthionitazene | 4-methylthio | 50 | 162623790 | 102471-37-4 |
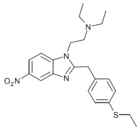
|
Ethylthionitazene | 4-ethylthio | 30 | 162623931 | 102758-70-3 |
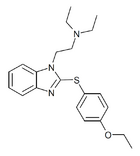
|
Etodesnitazene phenylthio analogue | 4-ethoxy | 1 | 21045 | |
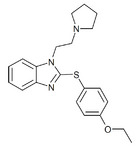
|
Etodesnitazene phenylthio / pyrrolidine analogue | 4-ethoxy | 2 | 19846499 |
See also
- 25-NB
- Arylcyclohexylamine
- List of aminorex analogues
- List of benzodiazepines
- List of fentanyl analogues
- List of phenyltropanes
- Structural scheduling of synthetic cannabinoids
- Substituted cathinone
References
- ↑ "DARK Classics in Chemical Neuroscience: Etonitazene and Related Benzimidazoles". ACS Chemical Neuroscience 12 (7): 1072–1092. April 2021. doi:10.1021/acschemneuro.1c00037. PMID 33760580.
- ↑ "Acute Intoxications and Fatalities Associated With Benzimidazole Opioid (Nitazene Analog) Use: A Systematic Review". Therapeutic Drug Monitoring 44 (4): 494–510. August 2022. doi:10.1097/FTD.0000000000000970. PMID 35149665.
- ↑ & Alfred Hunger"BENZMDAZOLES" US patent 2935514, published 1960-05-03, assigned to Ciba Pharmaceutical Products Inc.
- ↑ Drug Enforcement Administration (June 2021). "Benzimidazole Opioids". https://www.deadiversion.usdoj.gov/drug_chem_info/benzimidazole-opioids.pdf.
- ↑ 5.0 5.1 "A Forward-Thinking Approach to Addressing the New Synthetic Opioid 2-Benzylbenzimidazole Nitazene Analogs by Liquid Chromatography-Tandem Quadrupole Mass Spectrometry (LC-QQQ-MS)". Journal of Analytical Toxicology 46 (3): 221–231. March 2022. doi:10.1093/jat/bkab117. PMID 34792157.
- ↑ European Monitoring Centre for Drugs Drug Addiction (2020-11-13). "Report on the risk assessment of N,N-diethyl-2- 4-(1-methylethoxy)phenylmethyl]-5-nitro-1Hbenzimidazole- 1-ethanamine (isotonitazene) in accordance with Article 5c of Regulation (EC) No 1920/2006 (as amended)."]. European Monitoring Centre for Drugs and Drug Addiction (Publications Office of the European Union). doi:10.2810/107576. ISBN 9789294974952. https://op.europa.eu/en/publication-detail/-/publication/85c1a80f-27bf-11eb-9d7e-01aa75ed71a1. Retrieved 9 May 2022.
- ↑ Hoffman K, Hunger A, "Certain Alpha (1-diethylaminoethyl (2), Alpha Aryl Acetamides", US patent 2944062, issued 5 July 1960, assigned to Ciba Pharma Products Inc.
- ↑ "[Benzimidazole derivatives with strong analgesic effects]". Experientia 13 (10): 401–3. October 1957. doi:10.1007/BF02161117. PMID 13473818.
- ↑ "Synthesis, chemical characterization, and µ-opioid receptor activity assessment of the emerging group of nitazene new synthetic opioids.". Authorea. 12 November 2020. doi:10.22541/au.160520665.59016513/v1.
- ↑ "NOpiates: Novel Dual Action Neuronal Nitric Oxide Synthase Inhibitors with μ-Opioid Agonist Activity". ACS Medicinal Chemistry Letters 3 (3): 227–31. March 2012. doi:10.1021/ml200268w. PMID 24900459.
- ↑ "[Synthesis of analgesically active benzimidazole derivatives with basic substitutions"]. Experientia 13 (10): 400–1. October 1957. doi:10.1007/BF02161116. PMID 13473817. https://www.scribd.com/doc/78362960/Synthese-basisch-substituierter-analgetisch-wirksamer-Benzimidazol-Derivate-Synthesis-of-analgesically-active-benzimidazole-derivatives-with-basic-s.
- ↑ "Benzimidazol-Derivate und verwandte Heterocyclen. IV. Die Kondensation von o-Phenylendiamin mit α-Aryl- und γ-Aryl-acetessigester" (in de). Helvetica Chimica Acta 43 (4): 1046–1056. 1960. doi:10.1002/hlca.19600430413. https://www.scribd.com/doc/78122927/Benzimidazole-Derivatives-and-Related-Hetero-Cycles-IV-the-Condensation-of-O-phenylenediamine-With-a-And-Gamma-Aryl-Acetoacetate-Helv-Chim-Acta-19.
- ↑ "Benzimidazol-Derivate und verwandte Heterocyclen V. Die Kondensation von o-Phenylendiamin mit aliphatischen und alicyclischen β-Ketoestern" (in de). Helvetica Chimica Acta 43 (5): 1298–1313. 1960. doi:10.1002/hlca.19600430515. https://www.scribd.com/doc/78122989/Benzimidazole-derivatives-and-related-heterocycles-V-The-condensation-of-o-phenylenediamine-with-aliphatic-and-alicyclic-%C3%9F-keto-esters-Helv-Chim-Ac.
- ↑ "Benzimidazol-Derivate und verwandte Heterocyclen VI. Synthese von Phenyl-[1-aminoalkyl-benzimidazolyl-(2)-essigsäure-estern und -amiden"] (in de). Helvetica Chimica Acta 43 (6): 1727–1733. 1960. doi:10.1002/hlca.19600430634. https://www.scribd.com/doc/78122995/Benzimidazole-Derivatives-and-Related-Hetero-Cycles-VI-Synthesis-of-Phenyl-1-Aminoalkyl-benzimidazolyl-2-Acetic-Acid-Esters-and-Amides-Helv-Ch.
- ↑ "Benzimidazol-Derivate und verwandte Heterocyclen VII. Synthese neuer 2-Amino-benzimidazole" (in de). Helvetica Chimica Acta 44 (5): 1273–1282. 1961. doi:10.1002/hlca.19610440513. https://www.scribd.com/doc/78123010/Benzimidazole-Derivatives-and-Related-Hetero-Cycles-VII-Synthesis-of-New-2-Amino-benzimidazole-Helv-Chim-Acta-1961-44-5-1273-1282.
- ↑ "[Benzimidazole derivatives with strong analgesic effects"]. Experientia 13 (10): 401–3. October 1957. doi:10.1007/BF02161117. PMID 13473818. https://www.scribd.com/doc/78362970/Uber-Benzimidazolderivate-mit-starker-analgetischer-Wirkung-Benzimidazole-derivatives-with-strong-analgesic-effects-%E2%80%93-F-Goss-H-Turrian-%E2%80%93-Experienti.
- ↑ "[Studies on 2-benzimidazolethiol derivatives. N. Analgesic effect and pharmacological property of 1-(2-diethylaminoethyl)-2-(p-ethoxyphenylthio)benzimidazole hydrochloride]" (in ja). Yakugaku Zasshi 87 (3): 296–301. March 1967. doi:10.1248/yakushi1947.87.3_296. PMID 6069375.
- ↑ "[Studies on 2-benzimidazolethiol derivatives. V. Structure-activity relationship on analgesic action of 1-(dialkylamino-alkyl)-2-(p-ethoxyphenylthio)benzimidazole]" (in ja). Yakugaku Zasshi 87 (3): 301–9. March 1967. doi:10.1248/yakushi1947.87.3_301. PMID 6069376.
- ↑ "[Studies on 2-benzimidazolethiol derivatives. VI. Synthesis and analgesic effect of 1-(2-diethylaminoethyl)-2-(p-ethoxyphenylthio)-5-substituted benzimidazole]" (in ja). Yakugaku Zasshi 89 (5): 617–26. May 1969. doi:10.1248/yakushi1947.89.5_617. PMID 5817995.
- ↑ "Toward a Universal μ-Agonist Template for Template-Based Alignment Modeling of Opioid Ligands". ACS Omega 4 (17): 17457–17476. October 2019. doi:10.1021/acsomega.9b02244. PMID 31656918.
- ↑ "Analgesics and their antagonists: biochemical aspects and structure-activity relationships". Progress in Medicinal Chemistry 4: 171–218. February 1965. doi:10.1016/s0079-6468(08)70169-3. ISBN 9780444533234. PMID 5319798.
- ↑ "Exploring the effectiveness of novel benzimidazoles as CB2 ligands: synthesis, biological evaluation, molecular docking studies and ADMET prediction". MedChemComm 9 (12): 2045–2054. December 2018. doi:10.1039/c8md00461g. PMID 30647880.
- ↑ Chimica Therapeutica 2(16): 1967.
- ↑ "Analysis of highly potent synthetic opioid nitazene analogs and their positional isomers". Drug Testing and Analysis. November 2022. doi:10.1002/dta.3415. PMID 36437623.